Annual Report 2010-2011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bachelor's Programme in English Language and Literature, St
Bachelor’s Programme in English Language and Literature, St. Teresa’s College (Autonomous) ST.TERESA’S COLLEGE (AUTONOMOUS) ERNAKULAM (Affiliated to Mahatma Gandhi University, Kottayam) CURRICULUM AND SYLLABI FOR BACHELOR’S PROGRAMME IN ENGLISH LANGUAGE AND LITERATURE AND SYLLABI FOR COMPLEMENTARY COURSES IN ENGLISH LANGUAGE AND LITERATURE Under Choice Based Credit & Semester System (2018 Admissions) Bachelor’s Programme in English Language and Literature, St Teresa’s College (Autonomous) ST. TERESA’S COLLEGE (AUTONOMOUS), ERNAKULAM DEPARTMENT OF ENGLISH BOARD OF STUDIES IN ENGLISH Sl Name of the Official Address Designation No. member 1 Dr. Tessy Anthony C. Associate Professor Chairman Department of English and Centre for Research, St. Teresa’s College. 2 Dr. Janaky Sreedharan Associate Professor Subject Expert Department of English, Calicut University 3 Dr. Meena T. Pillai, Associate Professor, Institute of Subject Expert English & Director, Centre for Cultural Studies, University of Kerala, Thiruvananthapuram, 4 Dr. Kalyani Vallath Director, Total English Solutions Industrial Expert 5 Ms. Alicen Jacob Assistant Professor, Alumni Aquinas College, Edakochi. 6 Dr. Beena Job, Associate Professor & Head Member Department of English and Centre for Research, St. Teresa’s College (Autonomous), Ernakulam 7 Dr. Latha R. Nair Associate Professor, Member Department of English, St. Teresa’s College (Autonomous), Ernakulam 8 Dr. Priya K. Nair Assistant Professor, Member Department of English, St. Teresa’s College (Autonomous), Ernakulam Curriculum and Syllabus 2018 admissions onwards 1 Bachelor’s Programme in English Language and Literature, St Teresa’s College (Autonomous) List of teachers who contributed to Board of Studies 1. Dr. Tessy Anthony C., Chairman, Board of Studies in English 2. -

Sl. No Name Desig Subject School Place District Dob Moa Rank Year Doa Dor Gen 1 K Sivakamai Bt Asst Tamil Ghss Thennilai Karu
01.01.2019 நிலவரப்ப அர / நகராட் ேமல்நிைலப்பள்ளிப் பட்டதாரி ஆரியர் / பள்ளித் ைண ஆய்வர் / வட்டார வளைமய பற்நர் பதல் இந் கைலயாரியர் (தழ்) பத உயர்ற் தவாய்ந்த நபர்களின் ெபயர் பட்யல் SL. NAME DESIG SUBJECT SCHOOL PLACE DISTRICT DOB MOA RANK YEAR DOA DOR GEN NO 1 K SIVAKAMAI BT ASST TAMIL GHSS THENNILAI KARUR 5/25/1969 9/20/1994 9/20/1994 F 2 DHANALAKSHMI J BT ASST TAMIL GHSS SUNDAPALAYAM COIMBATORE 6/9/1969 TRB 55 1996 12/6/1996 12/6/1996 F 3 M.KANCHANADEVI BT ASST TAMIL GHSS UPPIDAMANGALAM KARUR 7/17/1964 TRB 304 1996 12/6/1996 12/6/1996 F 4 PREMALATHA V BT ASST TAMIL SMA GHSS SIRUTHONDANALOOR THOOTHUKUDI 6/1/1963 TRB 77218 1998 8/20/1998 8/20/1998 F 5 VIJAYALAKSHMI C BT ASST TAMIL GHS EGUVARPALAYAM THIRUVALLUR 6/9/1965 TRB 77951 1999 12/9/1999 12/9/1999 F 6 RATHINAM T BT ASST TAMIL GHSS (G) NATHAM DINDIGUL 3/21/1967 TRB 77955 1999 12/8/1999 12/8/1999 F 7 P.MURUGAN BT ASST TAMIL GHSS VELLIAMPATTI MADURAI 2/21/1967 TRB 78054 1999 12/8/1999 12/8/1999 M 8 P.SHANBAKAVADIVU BT ASST TAMIL GHSS KELAMBAKKAM KANCHIPURAM 7/29/1963 TRB 78252 1999 12/13/1999 12/13/1999 F 9 R.SHYAMALA BT ASST Tamil GGHSS Mannachanallur Trichy 5/30/1966 TRB 78904 2000 11/3/2000 11/3/2000 F 10 V. THILAKAVATHI BT ASST TAMIL CCMA GHSS RAJA STREET, COIMBATORE 11/26/1962 PRO - 2000 11/3/2000 11/3/2000 F 11 S.KAVITHA BT ASST TAMIL GHSS EMANESWARAM RAMANATHAPURAM 2/17/1963 TRB 79015 2000 11/6/2000 11/6/2000 F 12 DAISY KALAVATHI E BT ASST TAMIL GHSS UDAYAPATTI SALEM 3/10/1962 TRB 79017 2000 11/7/2000 11/7/2000 F 13 K SELVI BT ASST TAMIL GHS U.AMMAPATTI THENI 6/15/1962 TRB 79070 2000 11/6/2000 11/6/2000 F 14 CHITRADEVI R BT ASST TAMIL GHS MANIYNJI MADURAI 4/24/1964 TRB 79081 2000 11/3/2000 11/3/2000 F 15 S.GOVINDARAJ B.T.ASST. -

Promising Signs Ahead Media & Entertainment in South India
Promising signs ahead Media & Entertainment in South India Media & Entertainment Business Conclave – Chennai October 2012 Contents Message from FICCI ............................................................................................................................................................... 3 Message from Deloitte Touche Tohmatsu India Pvt. Ltd. (Deloitte) ......................................................................................... 4 Message from Deloitte Touche Tohmatsu India Pvt Ltd (Deloitte)........................................................................................... 5 1. Introduction .................................................................................................................................................................... 6 2. Film .............................................................................................................................................................................. 11 3. Television .................................................................................................................................................................... 30 4. Print ............................................................................................................................................................................. 51 5. Radio ........................................................................................................................................................................... 64 6. Direct tax - -
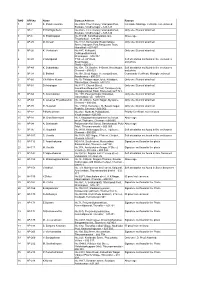
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -
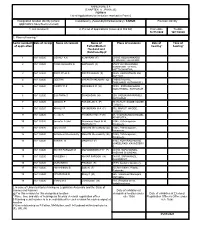
(CHAPTER V , PARA 25) FORM 9 List of Applications for Inclusion
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): TARUR Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 16/11/2020 16/11/2020 3. Place of hearing * Serial number$ Date of receipt Name of claimant Name of Place of residence Date of Time of of application Father/Mother/ hearing* hearing* Husband and (Relationship)# 1 16/11/2020 JISHNU K K KUMARAN (F) 09/566, KOLAVANKADU, PERINGOTTUKURUSSI, , 2 16/11/2020 PRABHAKARAN G GOPALAN (F) 65A/5 VRINDAVANAM, KUNNINMEL VEEDU, KOTTACHANTHA, , 3 16/11/2020 SREEJITHA S SREEDHARAN (F) 359/5, VARIYATHKALAM, KOTTAYI, , 4 16/11/2020 GEETHA VASANTHAKUMARI (O) THEKEPALAYIL, THOLANUR, KUTHANUR 2, , 5 16/11/2020 CHIPPY P S RAGESH K R (H) 05/35 SWPANAKOODU , NADUTHARA , KUTHANUR , , 6 16/11/2020 SUJITHRA S SIVADASAN (H) 209, VADUKANPARAMBU, KUTHANUR , , 7 16/11/2020 MANU K P PANGELAN K (F) 70, KURUTHICODE HOUSE, TARUR I, , 8 16/11/2020 ANJALY R RAVINDRAN M A (F) 502, MARUTHAKODE , TARUR I, , 9 16/11/2020 Keerthi K PRASANTHAN P (H) 23, VADAKKUMURI HOUSE, ATHIPOTTA, , 10 16/11/2020 Janeefar A Jani Ahammed Nissar M M 7/345, Pathanapuram, Musthafa (H) Kavassery, , 11 16/11/2020 Jaseena M Manaf M Meerankutty (O) 7/345, Pathanapuram, Kavassery, , 12 16/11/2020 Anfurdeen Meerankutty Manaf M Meerankutty (O) 7/345, Pathanapuram, Kavassery, , 13 16/11/2020 SANIKA M MADHU S (F) 9/144, KOKKRAD HOUSE VAKEELPADI, KAVASSERY -

MA English Revised (2016 Admission)
KANNUR Li N I \/EttSIl Y (Abstract) M A Programme in English Language programnre & Lirerature undcr Credit Based semester s!.stem in affiliated colieges Revised pattern Scheme. s,'rabus and of euestion papers -rmplemenred rvith effect from 2016 admission- Orders issued. ACADEMIC C SECTION UO.No.Acad Ci. til4t 20tl Civil Srarion P.O, Dared,l5 -07-20t6. Read : l. U.O.No.Acad/Ct/ u 2. U.C of €ven No dated 20.1O.2074 3. Meeting of the Board of Studies in English(pc) held on 06_05_2016. 4. Meeting of the Board of Studies in English(pG) held on 17_06_2016. 5. Letter dated 27.06.201-6 from the Chairman, Board of Studies in English(pc) ORDER I. The Regulations lor p.G programmes under Credit Based Semester Systeln were implernented in the University with eriect from 20r4 admission vide paper read (r) above dated 1203 2014 & certain modifications were effected ro rhe same dated 05.12.2015 & 22.02.2016 respectively. 2. As per paper read (2) above, rhe Scherne Sylrabus patern - & ofquesrion papers rbr 1,r A Programme in English Language and Literature uncler Credir Based Semester System in affiliated Colleges were implcmented in the University u,.e.i 2014 admission. 3. The meeting of the Board of Studies in En8lish(pc) held on 06-05_2016 , as per paper read (3) above, decided to revise the sylrabus programme for M A in Engrish Language and Literature rve'f 2016 admission & as per paper read (4) above the tsoard of Studies finarized and recommended the scheme, sy abus and pattem of question papers ror M A programme in Engrish Language and riterature for imprementation wirh efl'ect from 20r6 admissiorr. -

List of Books 2018 for the Publishers.Xlsx
LIST I LIST OF STATE BARE ACTS TOTAL STATE BARE ACTS 2018 PRICE (in EDITION SL.No. Rupees) COPIES AUTHOR/ REQUIRED PRICE FOR EDN / YEAR PUBLISHER EACH COPY APPROXIMATE K.G. 1 Abkari Laws in Kerala Rajamohan/Ar latest 898 5 4490 avind Menon Govt. 2 Account Code I Kerala latest 160 10 1600 Publication Govt. 3 Account Code II Kerala latest 160 10 1600 Publication Suvarna 4 Advocates Act latest 790 1 790 Publication Advocate's Welfare Fund Act George 5 & Rules a/w Advocate's Fees latest 120 3 360 Johnson Rules-Kerala Arbitration and Conciliation 6 Rules (if amendment of 2016 LBC latest 80 5 400 incorporated) Bhoo Niyamangal Adv. P. 7 latest 1500 1 1500 (malayalam)-Kerala Sanjayan 2nd 8 Biodiversity Laws & Practice LBC 795 1 795 2016 9 Chit Funds-Law relating to LBC 2017 295 3 885 Chitty/Kuri in Kerala-Laws 10 N Y Venkit 2012 160 1 160 on Christian laws in Kerala Santhosh 11 2007 520 1 520 Manual of Kumar S Civil & Criminal Laws in 12 LBC 2011 250 1 250 Practice-A Bunch of Civil Courts, Gram Swamy Law 13 Nyayalayas & Evening 2017 90 2 180 House Courts -Law relating to Civil Courts, Grama George 14 Nyayalaya & Evening latest 130 3 390 Johnson Courts-Law relating to 1 LIST I LIST OF STATE BARE ACTS TOTAL STATE BARE ACTS 2018 PRICE (in EDITION SL.No. Rupees) COPIES AUTHOR/ REQUIRED PRICE FOR EDN / YEAR PUBLISHER EACH COPY APPROXIMATE Civil Drafting and Pleadings 15 With Model / Sample Forms LBC 2016 660 1 660 (6th Edn. -

Women and Political Change in Kerala Since Independence
WOMEN AND POLITICAL CHANGE IN KERALA SINCE INDEPENDENCE THESIS SUBMITTED TO THE COCHIN UNIVERSITY or SCIENCE AND TECHNOLOGY FOR THE AWARD or THE DEGREE or DOCTOR OF PHILOSOPHY UNDER THE FACULTY or SOCIAL SCIENCES BY KOCHUTHRESSIA, M. M. UNDER THE SUPERVISION OF DR. J. T. PAYYAPPILLY PROFESSOR SCHOOL OF MANAGEMENT STUDIES COCHIN UNIVERSITY OF SCIENCE AND TECHNOLOGY COCHIN - 682 022, KERALA October 1 994 CERTIFICATE Certified that the thesis "Women and Political Change in Kerala since Independence" is the record of bona fide research carried out by Kochuthressia, M.M. under my supervision. The thesis is worth submitting for the degree of Doctor of Philosophy under the Faculty of Social Sciences. 2’/1, 1 :3£7:L§¢»Q i9¢Z{:;,L<‘ Professorfir.J.T.§ay§a%pilly///// ” School of Management Studies Cochin University of Science and Technology Cochin 682 022 Cochin 682 022 12-10-1994 DECLARATION I declare that this thesis is the record of bona fide research work carried out knrxme under the supervision of Dr.J.T.Payyappilly, School (HS Management. Studies, Cochin University of Science and Technology, Cochin 682 022. I further declare that this thesis has not previously formed the basis for the award of any degree, diploma, associateship, fellowship or other similar title of recognition. ¥E;neL£C-fl:H12§LJJ;/f1;H. Kochuthfe§§ia7—§iM. Cochin 682 022 12-10-1994 ACKNOWLEDGEMENTS Once the topic "Women and Political Change in Kerala since Independence" was selected for the study, I received a lot of encouragement from many men and women who'are genuinely concerned about the results (M5 gender discrimination. -

MPPSC PRELIMS the Only Comprehensive “CURRENT AFFAIRS” Magazine of “MADHYA PRADESH”In “ENGLISH MEDIUM”
MPPSC PRELIMS The Only Comprehensive “CURRENT AFFAIRS” Magazine of “MADHYA PRADESH”in “ENGLISH MEDIUM” National International MADHYA CURRENT Economy PRADESH MP Budget Current Affairs AFFAIRS MP Eco Survey MONTHLY Books-Authors Science Tech Personalities & Environment Sports OCTOBER 2020 Contact us: mppscadda.com [email protected] Call - 8368182233 WhatsApp - 7982862964 Telegram - t.me/mppscadda OCTOBER 2020 (CURRENT AFFAIRS) 1 MADHYA PRADESH NEWS Best wishes on International day of Older Persons Chief Minister Shri Shivraj Singh Chouhan has extended his best wishes on the International Day of Older Persons. He said that the elderly or senior persons have life experiences. They have the capacity to resolve many complicated problems. The biggest thing is that our elders have the qualities of patience, humility, ability, decision making and above all, the acquired knowledge that can give a direction to the society. Our youth must respect the elders. Their teachings must be imbibed in our lives. Chief Minister said that the elders are our heritage. Many legal provisions have been made for their honour and protection. Chief Minister has extended his best wishes to all senior citizens and elders on Senior Citizens day. Photos telling the story of Corona period Chief Minister Shri Shivraj Singh Chouhan today awarded the winners of the state level photo contest based on Covid-19 in a programme organized at Manas Bhawan and congratulated the photographers. Also inaugurated an exhibition of photographs clicked by press photographers of the state during the Corona period. Chief Minister Shri Chouhan said that the creativity of the photographers during Covid-19 crisis is apparent in this exhibition. -

Higher Secondary: SET
RRB PSC Higher Secondary: SET 1. The 190th member of United Nations 6. The world’s biggest Information Technology and tele- (a) Switzerland (b) East Timor communications trade fair-Ce BIT was held in (c) Tuvalu (d) None of these (a) Hannover (b) Shanghai 2. The first American Woman to climb the Everest from (c) New York (d) None of these both North and South faces 7. Gone with the Wind is a book written by (a) Susan Ershler (b) Ellen Miller (a) Rajani Kothari (b) Margaret Mitchell (c) Ang Rita (d) None of these (c) Sheila Gujral (d) None of these 3. The retired IAS officer who has been appointed as the 8. The first summit of the Conference on Interaction chief co-ordinator of relief operations in Gujarat and Confidence Building Measures in Asia (CICA) (a) K.P.S. Gill (b) S.M.F. Bokhari was held in (a) Beijing (b) Kathmandu (c) Sudha Murthy (d) None of these (c) Almatty (d) None of these 4. The Indian Army Chief who has been conferred the title 9. The Indian who has been appointed as the Under-Sec- of honorary general of the Royal Nepalese Army by retary General for Public Information in the United Na- king Gyanendra tions. (a) A.R. Gandhi (b) S. Padmanabhan (a) R.K. Laxman (b) Shashi Tharoor (c) S. Krishnaswamy (d) None of these (c) B.P. Singh (d) None of these 5. The winner of the Miss Universe title 2002 10. The newly appointed President of ‘FIFA’ (a) Oxana Fedorova (b) Justine Pasek (a) Jacques Rogge (b) Sepp Blatter (c) Ling Zhou (d) None of these (c) Sen Ruffianne (d) None of these Answers & Explanations 1. -

Library Catalogue
Id Access No Title Author Category Publisher Year 1 9277 Jawaharlal Nehru. An autobiography J. Nehru Autobiography, Nehru Indraprastha Press 1988 historical, Indian history, reference, Indian 2 587 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence historical, Indian history, reference, Indian 3 605 India from Curzon to Nehru and after Durga Das Rupa & Co. 1977 independence 4 3633 Jawaharlal Nehru. Rebel and Stateman B. R. Nanda Biography, Nehru, Historical Oxford University Press 1995 5 4420 Jawaharlal Nehru. A Communicator and Democratic Leader A. K. Damodaran Biography, Nehru, Historical Radiant Publlishers 1997 Indira Gandhi, 6 711 The Spirit of India. Vol 2 Biography, Nehru, Historical, Gandhi Asia Publishing House 1975 Abhinandan Granth Ministry of Information and 8 454 Builders of Modern India. Gopal Krishna Gokhale T.R. Deogirikar Biography 1964 Broadcasting Ministry of Information and 9 455 Builders of Modern India. Rajendra Prasad Kali Kinkar Data Biography, Prasad 1970 Broadcasting Ministry of Information and 10 456 Builders of Modern India. P.S.Sivaswami Aiyer K. Chandrasekharan Biography, Sivaswami, Aiyer 1969 Broadcasting Ministry of Information and 11 950 Speeches of Presidente V.V. Giri. Vol 2 V.V. Giri poitical, Biography, V.V. Giri, speeches 1977 Broadcasting Ministry of Information and 12 951 Speeches of President Rajendra Prasad Vol. 1 Rajendra Prasad Political, Biography, Rajendra Prasad 1973 Broadcasting Eminent Parliamentarians Monograph Series. 01 - Dr. Ram Manohar 13 2671 Biography, Manohar Lohia Lok Sabha 1990 Lohia Eminent Parliamentarians Monograph Series. 02 - Dr. Lanka 14 2672 Biography, Lanka Sunbdaram Lok Sabha 1990 Sunbdaram Eminent Parliamentarians Monograph Series. 04 - Pandit Nilakantha 15 2674 Biography, Nilakantha Lok Sabha 1990 Das Eminent Parliamentarians Monograph Series. -

Malankara Mar Thoma Syrian Church SABHA PRATHINIDHI MANDALAM 2017 - 2020 Address List of Mandalam Members Report Date: 27/07/2017 DIOCESE - ALL Page 1 of 46
Malankara Mar Thoma Syrian Church SABHA PRATHINIDHI MANDALAM 2017 - 2020 Address List of Mandalam Members Report Date: 27/07/2017 DIOCESE - ALL Page 1 of 46 L001 (NORTH CAROLINA MTC) L002 (LUBBOCK EMMANUEL) L003 (ATLANTA HERMON) MRS. VIJI MATHEW DR. P.JOHN LINCOLN MR. SAGIN K.MAMMAN 12700, RICHMOND RUN DRIVE 2404 YORK AVENUE 1960 SPRING MIST TERRACE RALEIGH, NORTH CAROLINA, 27614 LUBBOCK, TEXAS 79407 LAWRENCE VILLE, GA - 30043 U.S.A U.S.A U.S.A 919-562-8167, 919-795-8409 8067976000, 8064415131 6783760015, 404 229 7054 [email protected] [email protected] [email protected] L004 (TORONTO ST MATHEWS) L005 (BOSTON CARMEL) L006 (CHICAGO ST THOMAS) MR. JACOB JOSEPH MRS. MOLLY KURIAN MR. C.VARUGHESE PHILIP 2507 GRAND OAK TRAIL, OAK VILLE 43 DELANEY STREET,STOW, 1481 AUTUMN TRL. ONTARIO, CANADA- L6MOR7 MA- 01775 ADDISON, IL-60101 001 289 8373171, 001 905 399 6180 U.S.A U.S.A [email protected] 978 897 1260,978 793 1711 630-250-0619, 630-222-7021 [email protected] [email protected] L007 (PHILADELPHIA BETHEL) L008 (CONNECTICUT JERUSALEM) L009 (AUSTIN MTC) MRS. DEENAMMA THOMAS MR. MATHEWS THOMAS MR. SABU T.CHERIYAN 1137 ALTON PLACE 19 CEDAR ST. 2316 PARADISE RIDGE DR. PHILADELPHIA, P.A - 19115 DANBURY, CT-06811 ROUND ROCK, U.S.A U.S.A TEXAS 76665-7911, U.S.A 215 342 0237, 2672707974 203 205 0659, 203 312 4105 512 341 8084, 512 468 4457 [email protected] [email protected] [email protected] L010 (KATTANAM ST THOMAS) L011 (EDMONTON TRINITY) L012 (SALEM M.T.C EASTERN LONG EVANG.