Cr Vi Cancer 4Dec Clean
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exposure to Carcinogens and Work-Related Cancer: a Review of Assessment Methods
European Agency for Safety and Health at Work ISSN: 1831-9343 Exposure to carcinogens and work-related cancer: A review of assessment methods European Risk Observatory Report Exposure to carcinogens and work-related cancer: A review of assessment measures Authors: Dr Lothar Lißner, Kooperationsstelle Hamburg IFE GmbH Mr Klaus Kuhl (task leader), Kooperationsstelle Hamburg IFE GmbH Dr Timo Kauppinen, Finnish Institute of Occupational Health Ms Sanni Uuksulainen, Finnish Institute of Occupational Health Cross-checker: Professor Ulla B. Vogel from the National Working Environment Research Centre in Denmark Project management: Dr Elke Schneider - European Agency for Safety and Health at Work (EU-OSHA) Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers, or these calls may be billed. More information on the European Union is available on the Internet ( 48TU http://europa.euU48T). Cataloguing data can be found on the cover of this publication. Luxembourg: Publications Office of the European Union, 2014 ISBN: 978-92-9240-500-7 doi: 10.2802/33336 Cover pictures: (clockwise): Anthony Jay Villalon (Fotolia); ©Roman Milert (Fotolia); ©Simona Palijanskaite; ©Kari Rissa © European Agency for Safety and Health at Work, 2014 Reproduction is authorised provided the source is acknowledged. European Agency for Safety and Health at Work – EU-OSHA 1 Exposure to carcinogens and work-related cancer: -

Occupational Cancer Risk Series Diesel Engine Exhaust
Occupational Cancer Risk Series Diesel engine exhaust There may be hazards where you work that increase your risk of developing cancer. This factsheet discusses occupational hazards related to diesel engine exhaust (DEE). Key messages • fuel used (e.g. low-sulphur diesel) • use of emission control system/s • In Australia, it is estimated that 1.2 million workers from many jobs are exposed to diesel • state of engine tuning and maintenance engine exhaust (DEE). • pattern of use (load and acceleration) • DEE contains airborne chemicals that are known • length of time the worker is exposed to cause cancer (carcinogens). Effective controls • Eliminate or reduce exposure to carcinogens by All Australian workplaces must follow work health using recommended controls. and safety laws; however these vary slightly • Refer to Safe Work Australia’s Guidance for between states and territories, but the duty of care Managing the Risks of Diesel Exhaust for more for employers and responsibilities of workers across information or contact your state or territory Australia is similar: work health and safety regulator. • Employers are required to ensure the health and Diesel engine exhaust and cancer safety of their workers at their workplace. DEE is created by burning diesel fuels. It contains a • Employers are required to ensure the health and mixture of airborne chemicals that can be harmful safety other people due to the work carried out. to people. When breathed in, these chemicals • Employers have a duty to control the risks increase your risk of developing long-term health associated with work. problems. This includes lung cancer and possibly bladder cancer. -

10 Facts You Should Know About Occupational Carcinogens
10 facts you should know about occupational carcinogens Carcinogens are the “time bombs” of hazardous substances in the workplace. But many substances do not develop their deadly effect until years after expo- sure. It’s a risk for the worker that is often underestimated – and represents an enormous challenge for industrial hygienists. The good news is that occup- ational cancer can be prevented through monitoring and protective measures. © Drägerwerk AG & Co. KGaA 1 10 FACTS ABOUT OCCUPATIONAL CARCINOGENS 1. Cancer is a major health hazard in the workplace. Cancer in the workplace is twice as common as occupational accidents. Every year, 660,000 deaths occur worldwide due to work-related cancer.1 2. Plastic is a deadly threat. 3. Carcinogens are toxic to cells. For many years, vinyl chloride was considered to be safe. This com- Carcinogenic substances are a subgroup of toxic agents, which have pound of carbon, hydrogen and chlorine is a raw material used in the the potential to cause cancer in living tissues. Carcinogen exposure production of PVC. In 1974, seven cases of severe (and very rare) can occur from the inhalation, ingestion, or absorption of many dif- liver cancer were found in individuals who worked at a PVC plant in ferent types of substances in our bodies. Louisville, Kentucky. Five of the afflicted workers, all of whom had Carcinogens may increase the risk of cancer by altering cellular worked with vinyl chloride for 20 years, had already died.2 metabolism or damaging substances such as proteins, ribonucleic It is only because of the efforts of the National Institute for Occup- acids, and especially DNA directly in cells – which interferes with ational Safety and Health (NIOSH) and the energetic probing of a biological processes. -

Overview of Occupational Cancer in Painters in Korea Jun-Pyo Myong1,2, Younmo Cho1, Min Choi1 and Hyoung-Ryoul Kim1,2*
Myong et al. Annals of Occupational and Environmental Medicine (2018) 30:10 https://doi.org/10.1186/s40557-018-0222-3 REVIEW Open Access Overview of occupational cancer in painters in Korea Jun-Pyo Myong1,2, Younmo Cho1, Min Choi1 and Hyoung-Ryoul Kim1,2* Abstract Comprehensive consideration is necessary for setting guidelines to evaluate evidence of occupational cancer in painters due to work-related exposure to carcinogens in paint (a phenomenon termed herein as “work-relatedness”). The aim of the present research is to perform a comprehensive review and to suggest criteria for the provision of compensation for occupational neoplasm among painters in Korea. In order to perform a comprehensive review, this study assessed and evaluated scientific reports of carcinogenicities from the International Agency for Research on Cancer (IARC) and the Industrial Injuries Advisory Council (IIAC), as well as reviewed the existing literature about occupational exposure among painters in Korea and the epidemiologic investigations of claimed cases of cancer among painters in Korea. The IARC declares that occupational exposures in commercial painting are classified as Group 1 carcinogens for lung cancer and bladder cancer among painters. The epidemiologic studies show consistent causal relationships between occupational exposure in painters and cancers such as lung cancer [meta relative risk: 1.34 (95% confidence intervals (CIs): 1.23-1.41)] and bladder cancer [meta relative risk: 1.24 (95% CIs: 1.16-1.33)]. In reviewing occupational cancer risks for commercial painters, the Industrial Injuries Advisory Council (IIAC) confirms occupational cancer risks for lung and bladder cancer among commercial painters. According to the IIAC, however, the elevated cancer risks reported in existing literature are not doubled in either lung or bladder cancer in commercial painters relative to the risks of these cancers in the general population. -
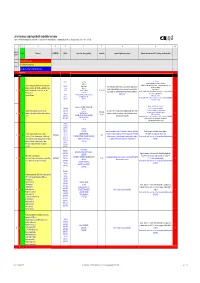
List of Substances Subject to Prohibited / Undesirable / Declaration
List of substances subject to prohibited / undesirable / declaration Appendix 1 to the Guidelines to Design and Purchasing Prohibition of Harmful Substances : ebmpapstMulfingen: 90-001 ebmpapstSt.Georgen: WN 3-12.2 ebmpapstLandshut: 60118.00040 (Ver. 5.0-31.07.2008) 1 2 3 4 5 6 7 8 9 10 Consecu N - new Substance EU-INDEX-No.: CAS-No. Source (law, decree, guideline). Hazard/risk example of application/occurrence Remarks and comments (of 0.1% deviating consideration limit) tive No. V = prohibited (verboten) U = undesirable (unerwünscht) D = subject to declaration (deklarationspflichtig) V = prohibited RoHS from 100 ppm 7439-92-1 TRGS 905, Pb-stabilisers and pigments are subject to declaration. GefStoffV, Prohibited: Anhydrite neutral lead carbonate, lead hydrogen carbonate and lead Lead and compounds (red lead oxide, lead sulphate, lead (7446-14-2 ChemVerbotsV, cable coating, heat transfer medium for accumulators, hybrid integrated sulphate as dye pigment hydrogen carbonate, lead carbonate, lead phtalates, lead 1319-46-6 BatterieV, circuits, stabilisers in plastics, vessels and pipes for aggressive liquids, < 0.4 % in batteries 1 V N acetates, lead phosphates, lead stereates , etc) lead 598-63-0 2002/95/EG (RoHS), K3, R 1, R 3 V E F according to RoHS: Lead as alloying additive in aluminum (up to 0.4 mass%) in steel (up to 67/458/EEC pigment production, lead alloys, anti-corrosion agents (fuel additives), chromate: refer to 0.35 mass%), in copper (up to 4%), 301-04-2 76/769/EEC (89/667/EEC, 97/10/EC, 97/56/EC) soldering agent chromium (VI) -

Diesel Engine Exhaust Burden of Occupational Cancer Fact Sheet
Diesel Engine Exhaust Burden of Occupational Cancer Fact Sheet WHAT IS DIESEL ENGINE EXHAUST? The combustion of diesel fuel in engines produces diesel engine exhaust, a complex mixture of gases and particulates. This mixture can contain other known and suspected carcinogens such as benzene, polycyclic aromatic hydrocarbons (PAHs), metals, and particulate matter. The composition of the mixture depends on a number of factors including the type of engine (heavy or light duty), the type of fuel and oil, sulphur levels, speed and load of operation, and emission control systems. The International Agency for Research on Cancer classifies diesel engine exhaust as a known carcinogen (IARC 1). WHAT ARE ITS HEALTH EFFECTS? • Lung cancer • Light-headedness, nausea, cough, and phlegm • Bladder cancer (suspected) • Allergic reactions • Irritation to eyes, throat, and bronchi THE BURDEN OF CANCER FROM WORKPLACE EXPOSURE TO DIESEL EXHAUST IN CANADA The term ‘burden’ refers to the human impact (deaths, illness, years of life lost) and the economic costs (health care, productivity) associated with a cause or group of causes of disease. Approximately 560 lung cancers and possibly 200 suspected bladder 560 cancers are due to to occupational exposure to diesel engine exhaust Lung cancers due each year in Canada, based on past exposures (1961-2001). This amounts to workplace diesel to 2.4% of lung cancer cases and 2.7% of suspected bladder cancer exhaust exposure cases diagnosed annually. WHAT IS THE ECONOMIC IMPACT? Work-related diesel engine exhaust exposure resulted in approximately $684 million in costs for newly diagnosed lung and suspected bladder cancer cases in 2011. -

An Overview of the Evidence on Environmental and Occupational Determinants of Cancer
AN OVERVIEW OF THE EVIDENCE ON ENVIRONMENTAL AND OCCUPATIONAL DETERMINANTS OF CANCER I.-BACKGROUND AND RATIONALE Cancer is a multifactorial disease due to a combined effect of genetic and external factors acting concurrently and sequentially. Overwhelming evidence indicates that the Cancer is a generic term for a large group of predominant contributor to many types of cancer is the diseases that can affect any part of the body. Other terms environment [9]. This finds support from multi-generation used are malignant tumours and neoplasms. One defining migrants studies showing adoption to the cancer risk of the feature of cancer is the creation of abnormal cells that grow host country [10, 11]. In addition, other studies with beyond their usual boundaries, and which can then invade identical twins have shown the role of the environment in adjoining parts of the body and spread to other organs. This the development of cancer and that genes are not the main process is referred to as metastasis [1]. explanation [12]. For instance, the concordance for breast cancer in twins was found to be only 20% [13]. The Cancer is the second most common cause of death combination of environmental exposures with some gene worldwide after cardiovascular diseases [2, 3]. Globally, polymorphisms may be synergistic and contribute to a there were reported 12.7 million new cases of cancer in substantial proportion of the cancer burden in the general 2008 (6,639,000 in men and 6,038,000 in women) and 7.6 population. million deaths due to cancer (4,225,000 in men and 3,345,000 in women) [4, 5]. -

Estimation of Occupational Exposure to Asbestos in Italy by the Linkage of Mesothelioma Registry (Renam) and National Insurance Archives
International Journal of Environmental Research and Public Health Article Estimation of Occupational Exposure to Asbestos in Italy by the Linkage of Mesothelioma Registry (ReNaM) and National Insurance Archives. Methodology and Results Chiara Airoldi 1, Daniela Ferrante 1, Lucia Miligi 2, Sara Piro 2, Giorgia Stoppa 2, Enrica Migliore 3, Elisabetta Chellini 4, Antonio Romanelli 5, Carlo Sciacchitano 6, Carolina Mensi 7 , Domenica Cavone 8 , Elisa Romeo 9, Stefania Massari 10, Alessandro Marinaccio 10 and Corrado Magnani 1,11,* 1 Unit of Medical Statistics and Cancer Epidemiology, Department of Translational Medicine, University of Eastern Piedmont, Novara, CPO-Piedmont, 28100 Novara, Italy; [email protected] (C.A.); [email protected] (D.F.) 2 Tuscany Regional Operating Centre of Low Etiological Fraction Occupational Cancer, Occupational and Environmental Epidemiology Branch, Cancer Risk Factors and Lifestyle Epidemiology Unit, Institute for Cancer Research, Prevention and Clinical Network (ISPRO), 50141 Florence, Italy; [email protected] (L.M.); [email protected] (S.P.); [email protected] (G.S.) 3 Piedmont Regional Operations Center of the National Mesothelioma Registry, Unit of Cancer Epidemiology, CPO-Piedmont and University of Turin, 10126 Turin, Italy; [email protected] 4 Tuscan Occupational Cancer Registry and Tuscan Mesothelioma Registry, Occupational and Environmental Epidemiology Branch, Cancer Risk Factors and Lifestyle Epidemiology Unit, Institute for Cancer Research, Prevention and Clinical -

Occupational Cancers Are Avoidable
Occupational A WORKPLACE GUIDE What is cancer? Cancer is not a single disease with a single type of treatment. There are more than 200 different kinds of cancer affecting different parts of the body. Cancers occur when new cells start growing out of control and develop into a lump or tumour. These tumours can be either benign or malignant. If it is benign the cells do not spread to other parts of the body, but if it is malignant the tumour can spread beyond the original area. Cancer is the name given to a malignant tumour. If the tumour is left untreated, it may spread into the surrounding tissues. Sometimes cells break away from the original cancer and spread to other organs in the body through the bloodstream or lymphatic system. When the cancer cells reach a new area they go on dividing and form a new tumour. Cancers can develop for a wide range of reasons. These include exposure to radiation – both from radioactive materials and the sun – infection by certain viruses, a genetic defect, a weakened immune system, age, bad diet, and exposure to chemical carcinogens. Carcinogens damage cells and make them more likely to turn cancerous. There are a wide range of known carcinogens, including tobacco smoke, asbestos fibres, diesel exhaust, radiation, and a wide range of chemicals found in the workplace. Although some cancers seem to develop for no apparent reason, most are a result of exposure to a carcinogen, lifestyle issues, genetic defects, age or a combination of these. This booklet primarily deals with cancers caused through exposures that are a result of work. -

The Burden of Occupational Cancer in Great Britain RR595 Technical Annex 5: Bladder Cancer
Health and Safety Executive The burden of occupational cancer in Great Britain Technical Annex 5: Bladder cancer Prepared by Imperial College London and the Health and Safety Laboratory for the Health and Safety Executive 2007 RR595 Technical Annex 5 Health and Safety Executive The burden of occupational cancer in Great Britain Technical Annex 5: Bladder cancer Lesley Rushton & Sally Hutchings Imperial College London Department of Epidemiology and Public Health Faculty of Medicine St Mary’s Campus Norfolk Place London W2 1PG Terry Brown Health and Safety Laboratory Harpur Hill Buxton SK17 9JN The aim of this project was to produce an updated estimate of the current burden of occupational cancer specifically for Great Britain. The primary measure of the burden of cancer used was the attributable fraction (AF), ie the proportion of cases that would not have occurred in the absence of exposure. Data on the risk of the disease due to the exposures of interest, taking into account confounding factors and overlapping exposures, were combined with data on the proportion of the target population exposed over the period in which relevant exposure occurred. Estimation was carried out for carcinogenic agents or exposure circumstances that were classified by the International Agency for Research on Cancer (IARC) as Group 1 or 2A carcinogens with strong or suggestive human evidence. Estimation was carried out for 2004 for mortality and 2003 for cancer incidence for cancer of the bladder, leukaemia, cancer of the lung, mesothelioma, non melanoma skin cancer (NMSC), and sinonasal cancer. The proportion of cancer deaths in 2004 attributable to occupation was estimated to be 8.0% in men and 1.5% in women with an overall estimate of 4.9% for men plus women. -

Alenka Skerjanc
NEW EXPOSURES AND EVIDENCES RELATED TO OCCUPATIONAL CANCER Dr. Alenka Škerjanc EASOM Summer School Riga, 29.08.2019 PORTRAIT OF THE EU WORKFORCE Proportion of persons (aged 15-64) reporting exposure to risk factors for physical health, 2007 and 2013 (%) Source: Eurostat, 2017 OCCUPATIONAL ... OCCUPATIONAL DISEASEl : • result of an exposure to risk factors arising from work activity (ILO) • cases, to which occupational origin has been approved by the national occupational authorities RECOGNISED OCCUPATIONAL DISEASE: • vary with national legislations and compensation practices • no harmonization at EU level WORK RELATED DISEASE: • includes disease where work played a role Self reported exposures to hazards at work 2005 – 2015 Source: 6th EWCS, Eurofound, 2017 Chemical risks Disinfectants in hospitals • Metal cutting fluids • 120 000 chemicals on the EU-market + millions of mixtures • Sectors: chemical/pharma, textile, automotive, construction, cleaning, health care, beauty etc. Fumigants • Up to 50% of all recognized occupational diseases linked to chemical exposure • New risks: nanomaterials (i.e. Carbon nanotubes) OCCUPATIONAL CANCERS Source: Takala J, ETUI, 2015, based on WHO and ILO data Most frequent carcinogens at work In high income countries: cancers are the first cause of work related mortality Polycyclic Aromatic Hydrocarbons Formaldehyde Aromatic amines Tetraclorethylene Cytostatic drugs Asbestos Crystalline silica Chromium VI Cd Ni Mineral oils Diesel engine exhaust Wood dust Tabaco smoke Solar radiation Shift work NOCCA (Nordic -

Sd0000029 Evaluation of Chromfte Ore and The
SD0000029 EVALUATION OF CHROMFTE ORE AND THE OPTIMUM METHODS FOR INDUSTRIAL EXTRACTION OF CHROMIUM A TI1HSIS SUBMITTED BY Bakheit Mustafa Mohamed Salih IN CANDIDATURE FOR TIU:DI:GRFJ:OI MASTER OF SC1HNCT DEPARTMENT OF CHEMISTRY FACULTY OF SCIENCE (P.O.BOX 321) OCTOBER 1999 UNIVERSITY OF KHARTOUM 31/ 28 ABSTRACT Samples of chromite ore, collected from Gam and Cheikay mining area (Ingessana Hills) in east Sudan, were analysed to assess the chromium content. Methods for extraction and analysis of chromium metal were developed and established. Analysis were carried out using atomic absorption spectroscopy (AAS) to estimate the contents of chromium, iron, calcium, and magnesium. X-ray fluorescence (XRF) was used to evaluate the levels of chromium, iron, and calcium in the ore. Volumetric analysis was performed to assess chromium and iron, whilst gravimetric analysis was employed to measure the amounts of calcium, magnesium, aluminum and silicon present in the ore. The data was chemically and statistically analyzed to compare the results obtained by the given analytical methods. The results are in good agreement except iron oxide, which displayed a significantly different value when measured by x-ray fluorescence. The data obtained exhibited similarity in almost all cases, when compared with local and global researches, reports, and literature. The study has revealed the average contents of Cr2O3, FeO, CaO, MgO, A12O3, and SiO2 as 40.66. 11.96, 11.94. 0.36. 16.94, 11.45% respectively. MnO and NiO were detected in trace amounts, the corresponding levels in the ore being 72 and 27 ppm. The average chromium content in extracted potassium dichromate measured by using AAS, XRF, and volumetric methods was found to be 31.7%.