02. Answers-1.Cdx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {Tpw(NO)(Pme3)}
Reactivity and Functionalization of Naphthalene and Anthracene Complexes of {TpW(NO)(PMe3)} Laura Jessica Strausberg Baltimore, Maryland B.A., Hollins University, 2008 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia July, 2013 ii Abstract Chapter 1 introduces the organic chemistry of aromatic hydrocarbons, with attention paid to regiochemical outcomes of organic reactions. The binding of naphthalene and anthracene to metal complexes is discussed, along with organic transformations they undergo as a result of their complexation. The previous work on osmium and rhenium complexes of naphthalene from the Harman group is explored. Finally, some spectroscopic techniques for exploring the chemistry of {TpW(NO)(PMe3)} complexes of naphthalene and anthracene are introduced. Chapter 2 discusses the highly distorted allyl complexes formed from {TpW(NO)(PMe3)} and the exploration of their origin. Attempts at stereoselectively deprotonating these cationic complexes is also discussed. 2 Chapter 3 describes our study of TpW(NO)(PMe3)(3,4-η -naphthalene)’s ability to undergo a Diels-Alder reaction with N-methylmaleimide. A solvent study suggested that this reaction proceeds by a concerted mechanism. To probe the mechanism further, we synthesized a series of methylated and methoxylated naphthalene complexes and measured their rates of reaction with N-methylmaleimide compared to the parent complex. We found that 1- substitution on the naphthalene increased the rate of cycloaddition, even if the substituent was in the unbound ring, while 2-substitution slowed the reaction rate when in the bound ring. This information is consistent with a concerted mechanism, as a 2-substituted product would be less able to isomerize to form the active isomer for the cycloaddition to occur. -

Crystal Structure of Methyl 10-(Pyridin-4-Yl)-Anthracene-9
Z. Kristallogr. NCS 2018; 233(3): 441–443 Xiang Huang and Da-Bin Shi* Crystal structure of methyl 10-(pyridin-4-yl)- anthracene-9-carboxylate, C21H15NO2 Table 1: Data collection and handling. Crystal: Block, colorless Size: 0.30 × 0.20 × 0.10 mm Wavelength: Mo Kα radiation (0.71073 Å) µ: 0.09 mm−1 Diffractometer, scan mode: Bruker SMART, φ and ω-scans θmax, completeness: 27.6°, >99% N(hkl)measured, N(hkl)unique, Rint: 9199, 3516, 0.027 Criterion for Iobs, N(hkl)gt: Iobs > 2 σ(Iobs), 2700 N(param)refined: 218 Programs: Bruker programs [1], SHELX [2, 3] before stirring for 2 h at 65 °C. Crude 10-bromo-anthracene- 9-carboxylic acid was precipitated by adding the acetic acid solution to 800 mL of ice/water slush, followed by suc- https://doi.org/10.1515/ncrs-2017-0334 tion filtration. The residue on the filter was dissolved in Received October 31, 2017; accepted February 20, 2018; available 500 mL of a 5% aquaeus solution of K2CO3 followed by online March 6, 2018 gravity filtration to remove undissolved side products such as 9,10-dibromoanthracene. The filtrate was acidified with Abstract concentrated HCl to precipitate crude 10-bromo-anthracene- Aba a = C21H15NO2, orthorhombic, 2 (no. 41), 22.149(2) Å, 9-carboxylic acid, which was recrystallized from 100 mL b = c = V = 3 Z = 13.2899(12) Å, 10.6679(10) Å, 3140.2(5) Å , 8, ethanol to yield 5.76 g of yellow needles. R F = wR F2 = T = gt( ) 0.0418, ref( ) 0.0953, 296(2) K. -

ORGANIC CHEMISTRY- I (Nature of Title Bonding and Stereochemistry) Module No
Subject Chemistry Paper No. and Paper 1: ORGANIC CHEMISTRY- I (Nature of Title Bonding and Stereochemistry) Module No. and Module 8: Aromaticity of fused rings Title Module Tag CHE_P1_M8 CHEMISTRY PAPER 1: ORGANIC CHEMISTRY- I(Nature of Bonding and Stereochemistry) MODULE 8: Aromaticity of fused rings TABLE OF CONTENT 1. Learning Outcomes 2. Introduction 3. Classification of fused ring systems 4. Aromaticity in fused ring systems 4.1. Aromaticity of some benzenoid fused systems 4.1.1. Naphthalene 4.1.2. Anthracene 4.1.3. Phenanthrene 4.1.4. Resonance energy of fused ring systems 4.2. Aromaticity of some non-benzenoid fused systems 4.2.1. Azulenes 4.2.2. Oxaazulenaones 5. Other fused ring systems 5.1. Phenalene 5.2. Benzo cyclobutadiene 5.3. Ferrocene 6. Summary CHEMISTRY PAPER 1: ORGANIC CHEMISTRY- I(Nature of Bonding and Stereochemistry) MODULE 8: Aromaticity of fused rings 1. Learning Outcomes After studying this module, you shall be able to: Learn about the fused rings Understand that how fused rings are classified Learn about the aromaticity of the fused rings Understand aromaticity in the benzenoid and non-benzenoid fused ring systems Learn about some other special cases 2. Introduction As you are already aware that the aromatic compounds apparently contain alternate double and single bonds in a cyclic structure and resemble benzene in chemical behavior. Up till now we have discussed the aromaticity in monocyclic rings. In this module, we shall discuss about the aromaticity of fused rings. So, before starting with the aromaticity of fused rings first we should know what fused rings are. -

Polar Covalent Bonds: Electronegativity
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms is not symmetrical Bond Polarity and Electronegativity Symmetrical Covalent Bonds Polar Covalent Bonds C – C + - C – H C – O (non-polar) (polar) Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Inductive Effect: shifting of sigma bonded electrons in resppygonse to nearby electronegative atom The Periodic Table and Electronegativity C – H C - Br and C - I (non-pol)lar) (po l)lar) Bond Polarity and Inductive Effect Nonpolar Covalent Bonds: atoms with similar EN Polar Covalent Bonds: Difference in EN of atoms < 2 Ionic Bonds: Difference in EN > 2 C–H bonds, relatively nonpolar C-O, C-X bonds (more electronegative elements) are polar Bonding electrons shift toward electronegative atom C acqqppuires partial positive char g,ge, + Electronegative atom acquires partial negative charge, - Inductive effect: shifting of electrons in a bond in response to EN of nearby atoms Electrostatic Potential Maps Electrostatic potential maps show calculated charge distributions Colors indicate electron- rich (red) and electron- poor (blue ) reg ions Arrows indicate direction of bond polarity Polar Covalent Bonds: Net Dipole Moments Molecules as a whole are often polar from vector summation of individual bond polarities and lone-pair contributions Strongly polar substances soluble in polar solvents like water; nonpolar substances are insoluble in water. Dipole moment ( ) - Net molecular polarity, due to difference in summed charges - magnitude of charge Q at end of molecular dipole times distance r between charges = Q r, in debyes (D), 1 D = 3.336 1030 coulomb meter length of an average covalent bond, the dipole moment would be 1.60 1029 Cm, or 4.80 D. -

Heterocycles 2 Daniel Palleros
Heterocycles 2 Daniel Palleros Heterocycles 1. Structures 2. Aromaticity and Basicity 2.1 Pyrrole 2.2 Imidazole 2.3 Pyridine 2.4 Pyrimidine 2.5 Purine 3. Π-excessive and Π-deficient Heterocycles 4. Electrophilic Aromatic Substitution 5. Oxidation-Reduction 6. DNA and RNA Bases 7. Tautomers 8. H-bond Formation 9. Absorption of UV Radiation 10. Reactions and Mutations Heterocycles 3 Daniel Palleros Heterocycles Heterocycles are cyclic compounds in which one or more atoms of the ring are heteroatoms: O, N, S, P, etc. They are present in many biologically important molecules such as amino acids, nucleic acids and hormones. They are also indispensable components of pharmaceuticals and therapeutic drugs. Caffeine, sildenafil (the active ingredient in Viagra), acyclovir (an antiviral agent), clopidogrel (an antiplatelet agent) and nicotine, they all have heterocyclic systems. O CH3 N HN O O N O CH 3 N H3C N N HN N OH O S O H N N N 2 N O N N O CH3 N CH3 caffeine sildenafil acyclovir Cl S N CH3 N N H COOCH3 nicotine (S)-clopidogrel Here we will discuss the chemistry of this important group of compounds beginning with the simplest rings and continuing to more complex systems such as those present in nucleic acids. Heterocycles 4 Daniel Palleros 1. Structures Some of the most important heterocycles are shown below. Note that they have five or six-membered rings such as pyrrole and pyridine or polycyclic ring systems such as quinoline and purine. Imidazole, pyrimidine and purine play a very important role in the chemistry of nucleic acids and are highlighted. -

Coulomb Pairing Resonances in Multiple-Ring Aromatic Molecules
Coulomb pairing resonances in multiple-ring aromatic molecules D.L. Huber* Physics Department, University of Wisconsin-Madison, Madison, Wisconsin 53706, USA Abstract We present an analysis of pairing resonances observed in photo-double-ionization studies of CnHm aromatic molecules with multiple benzene-like rings. The analysis, which is based on the Coulomb pairing model, is applied to naphthalene, anthracene, phenanthrene, pyrene and coronene, all of which have six-member rings, and azulene which is comprised of a five-member and a seven-member ring. There is a high energy resonance at ~ 40 eV that is found in all of the molecules cited and is associated with paired electrons localized on carbon sites on the perimeter of the molecule, each of which having two carbon sites as nearest neighbors. The low energy resonance at 10 eV, which is found only in pyrene and coronene, is attributed to the formation of paired HOMO electrons localized on arrays of interior carbon atoms that have the point symmetry of the molecule with each carbon atom having three nearest neighbors. The origin of the anomalous increase in the doubly charged to singly charged parent-ion ratio that is found above the 40 eV resonance in all of the cited molecules except coronene is discussed. *Mailing address: Physics Department, University of Wisconsin-Madison, 1150 University Ave., Madison, WI 53711, USA; e-mail: [email protected] 1 1. Introduction Recent studies of photo-double-ionization in CnHm multiple-ring (polycylic) aromatic molecules have revealed the existence of anomalous resonances in the ratio of the cross sections of doubly charged parent ions to singly charged parent ions I(2+)/I(1+) [1-4]. -
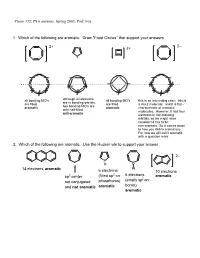
PS 6 Answers, Spring 2003, Prof
Chem 332, PS 6 answers, Spring 2003, Prof. Fox 1. Which of the following are aromatic. Draw "Frost Circles" that support your answers 2– 2+ 2+ + all bonding MO's although all electrons all bonding MO's this is an interesting case: this is are in bonding orbitals, are filled are filled a 4n+2 molecule, and it is flat-- two bonding MO's are aromatic aromatic characteristic of aromatic only half-filled molecules. However, it has four anti-aromatic electrons in non-bonding orbitals, so we might have considered this to be non-aromatic. So it comes down to how you define aromaticity. For now we will call it aromatic with a question mark. 2. Which of the following are aromatic. Use the Huckel rule to support your answer 2– P H B 14 electrons, aromatic H 6 electrons 10 electrons 2 6 electrons sp3 center (filled sp on aromatic (empty sp2 on not conjugated phosphorus) boron) and not aromatic aromatic aromatic 3. Compound 1 has an unusually large dipole moment for an organic hydrocarbon. Explain why (consider resonance structures for the central double bond) We need to remember that for any double bond compound, we can draw two polar resonance structure. Normally, these resonance structures are unimportant unimportant unimportant However, for 1 the story is different because there is a polar resonance form that has two aromatic components. In other words, 'breaking' the double bond gives rise to aromaticity, and therefore the polar resonance structure is important and leads to the observed dipole. dipole 1 8 e– 4 e– 6 e– 6 e– anti- anti- aromatic aromatic aromatic aromatic important resonance unimportant resonance structure structure 5. -

Anti-Coking Materials for Steam Crackers Copyright
ANTI-COKING MATERIALS FOR STEAM CRACKERS A Dissertation Presented to The Academic Faculty by Shilpa Mahamulkar In Partial Fulfillment of the Requirements for the Degree DOCTOR of PHILOSOPHY in the SCHOOL OF CHEMICAL AND BIOMOLECULAR ENGINEERING Georgia Institute of Technology May 2017 COPYRIGHT © 2017 BY SHILPA MAHAMULKAR ANTI COKING MATERIALS FOR STEAM CRACKERS Approved by: Prof. Christopher W. Jones, Advisor Prof. Athanasios Nenes School of Chemical & Biomolecular School of Earth & Atmospheric Engineering Sciences Georgia Institute of Technology Georgia Institute of Technology Prof. Pradeep K. Agrawal, Co-advisor Dr. Andrzej Malek School of Chemical & Biomolecular Hydrocarbons R&D, Midland Engineering The Dow Chemical Company Georgia Institute of Technology Prof. Thomas Fuller School of Chemical & Biomolecular Engineering Georgia Institute of Technology Date Approved: 20th March, 2017 To my wonderful parents Suresh and Vasanti Mahamulkar & my loving husband Ravi Kumar Kovvali ACKNOWLEDGEMENTS Foremost, I would like to extend my sincere thanks to my advisors Dr. Christopher Jones and Dr. Pradeep Agrawal for their continuous support throughout these five years. I am grateful for their invaluable advice, constructive criticism and the positive appreciation. Their enthusiasm and dedication towards work has been really inspiring. I thank the Dow Chemical Company, for funding the project and giving me an opportunity to acquire hands on experience in an industrial setting. I would like to thank our collaborators from University of Virginia, Prof. Robert Davis and Dr. Kehua Yin for the fruitful discussions and suggestions which have been instrumental in the work. I had the pleasure to work with Dr. Hyuk Taek Kwon and would like to thank him for mentoring me in a new field of coatings. -

Construction of Novel Molecular Architectures from Anthracene Units and Acetylene Linkers*
Pure Appl. Chem., Vol. 84, No. 4, pp. 917–929, 2012. http://dx.doi.org/10.1351/PAC-CON-11-09-07 © 2012 IUPAC, Publication date (Web): 9 February 2012 Construction of novel molecular architectures from anthracene units and acetylene linkers* Shinji Toyota‡ Department of Chemistry, Faculty of Science, Okayama University of Science, 1-1 Ridaicho, Kita-ku, Okayama 700-0005, Japan Abstract: To create novel π-conjugated compounds, we constructed various molecular archi- tectures from anthracene units and acetylene linkers. Several cyclic oligomers ranging from dimers to dodecamers were synthesized by macrocyclization of acyclic precursors with metal-catalyzed coupling reactions. The structures, dynamic behavior, and spectroscopic fea- tures were greatly influenced by the number of anthracene units and the combination of building units and linkers. Optically active and circular dichroism (CD)-active enantiomers of some chiral cyclic oligomers were resolved by chiral high-performance liquid chromato - graphy (HPLC). Conformational analysis of hexamers and higher oligomers was performed with the aid of density functional theory (DFT) calculations. Acyclic oligomers underwent reversible folding–unfolding processes via photochemical and thermal reactions. These results suggest that transannular π–π interactions between anthracene units are important fac- tors in controlling the structural and spectroscopic properties and functions of π-conjugated compounds. The scope and perspectives of this molecular design are discussed on the basis of previous studies. Keywords: aromatic compounds; alkynes; π–π interactions; stereochemistry; structure. INTRODUCTION In the chemistry of aromatic compounds, oligomeric structures consisting of simple repeating units are fascinating motifs for the creation of new compounds. The merits of this molecular design are the acces- sibility to a large number of compounds from simple building units as well as the ease of tuning elec- tronic properties by structural modifications. -

Structure of Benzene, Orbital Picture, Resonance in Benzene, Aromatic Characters, Huckel’S Rule B
Dr.Mrityunjay Banerjee, IPT, Salipur B PHARM 3rd SEMESTER BP301T PHARMACEUTICAL ORGANIC CHEMISTRY –II UNIT I UNIT I Benzene and its derivatives 10 Hours A. Analytical, synthetic and other evidences in the derivation of structure of benzene, Orbital picture, resonance in benzene, aromatic characters, Huckel’s rule B. Reactions of benzene - nitration, sulphonation, halogenationreactivity, Friedelcrafts alkylation- reactivity, limitations, Friedelcrafts acylation. C. Substituents, effect of substituents on reactivity and orientation of mono substituted benzene compounds towards electrophilic substitution reaction D. Structure and uses of DDT, Saccharin, BHC and Chloramine Benzene and its Derivatives Chemists have found it useful to divide all organic compounds into two broad classes: aliphatic compounds and aromatic compounds. The original meanings of the words "aliphatic" (fatty) and "aromatic" (fragrant/ pleasant smell). Aromatic compounds are benzene and compounds that resemble benzene in chemical behavior. Aromatic properties are those properties of benzene that distinguish it from aliphatic hydrocarbons. Benzene: A liquid that smells like gasoline Boils at 80°C & Freezes at 5.5°C It was formerly used to decaffeinate coffee and component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. A precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent in cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited. In 1970s it was associated with leukemia deaths. -
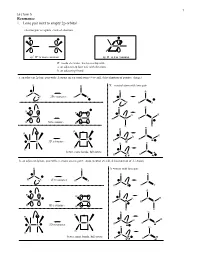
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

INVESTIGATION of POLYCYCLIC AROMATIC HYDROCARBONS (Pahs) on DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS
INVESTIGATION OF POLYCYCLIC AROMATIC HYDROCARBONS (PAHs) ON DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Ping Sun, M.S. ***** The Ohio State University 2004 Dissertation Committee: Approved by Professor Linda Weavers, Adviser Professor Harold Walker Professor Patrick Hatcher Adviser Professor Yu-Ping Chin Civil Engineering Graduate Program ABSTRACT The primary goal of this research was to examine polycyclic aromatic hydrocarbons (PAHs) on dry FGD by-products to determine environmentally safe reuse options of this material. Due to the lack of information on the analytical procedures for measuring PAHs on FGD by-products, our initial work focused on analytical method development. Comparison of the traditional Soxhlet extraction, automatic Soxhlet extraction, and ultrasonic extraction was conducted to optimize the extraction of PAHs from lime spray dryer (LSD) ash (a common dry FGD by-product). Due to the short extraction time, ultrasonic extraction was further optimized by testing different organic solvents. Ultrasonic extraction with toluene as the solvent turned out to be a fast and efficient method to extract PAHs from LSD ash. The possible reactions of PAHs under standard ultrasonic extraction conditions were then studied to address concern over the possible degradation of PAHs by ultrasound. By sonicating model PAHs including naphthalene, phenanthrene and pyrene in organic solutions, extraction parameters including solvent type, solute concentration, and sonication time on reactions of PAHs were examined. A hexane: acetone (1:1 V/V) ii mixture resulted in less PAH degradation than a dichloromethane (DCM): acetone (1:1 V/V) mixture.