Decomposition of Wood by Polypore Fungi in Tropics - Biological, Ecological and Environmental Factors- a Case Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
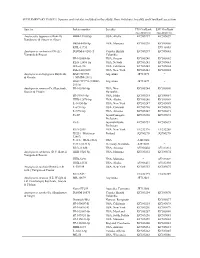
Suppl Table I
SUPPLEMENTARY TABLE I. Species and isolates included in the study, their vouchers, locality and GenBank accession Species Isolate number Locality ITS GenBank LSU GenBank accession no. accession no. Amylocystis lapponica (Romell) HHB-13400-Sp USA, Alaska KC585237 KC585059 Bondartsev & Singer ex Singer OKM-4418-Sp USA, Montana KC585238 KC585060 KHL-11755 − EU118603 Amyloporia carbonica (Overh.) DAOM-F-8281-T Canada, British KC585239 KC585061 Vampola & Pouzar Columbia FP-105585-Sp USA, Oregon KC585240 KC585062 RLG-12496-Sp USA, Nevada KC585241 KC585063 Wilcox-96 USA, California KC585242 KC585064 Zabel-40-GLN USA, New York KC585243 KC585065 Amyloporia nothofaginea Rajchenb. BAFC519794 Argentina JF713078 − & Gorjón (=MMBP-2011) BAFC519796 (=MBP- Argentina JF713079 − 2011a) Amyloporia sinuosa (Fr.) Rajchenb., FP-105386-Sp USA, New KC585244 KC585066 Gorjón & Pildain Hampshire FP-94464-Sp USA, Idaho KC585245 KC585067 HHB-12878-Sp USA, Alaska KC585246 KC585068 L-14130-Sp USA, New York KC585247 KC585069 L-6192-Sp USA, Colorado KC585248 KC585070 L-9792-Sp USA, Arizona KC585249 KC585071 Pa-3C JapanYamagata KC585250 KC585072 Prefecture Pa-3e JapanShizuoka KC585251 KC585073 Prefecture RLG-2538 USA, New York EU232196 EU232288 X725 (=Miettinen- Finland JQ700270 JQ700270 12407) P-115 (=RLG-2538) USA AJ416068 − P-211 (G-214) Germany, Karlsrube AJ345011 − RLG-1182R USA, Arizona AY966450 AY333831 Amyloporia sitchensis (D.V. Baxter) HHB-5320-Sp USA, Montana KC585252 KC585074 Vampola & Pouzar HHB-5298 USA, Montana − AY333829 HHB-12513 USA, Alaska AY966451 AY333830 -

Diversity of Polyporales in the Malay Peninsular and the Application of Ganoderma Australe (Fr.) Pat
DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS MOHAMAD HASNUL BIN BOLHASSAN FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS MOHAMAD HASNUL BIN BOLHASSAN THESIS SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF BIOLOGICAL SCIENCES FACULTY OF SCIENCE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 UNIVERSITI MALAYA ORIGINAL LITERARY WORK DECLARATION Name of Candidate: MOHAMAD HASNUL BIN BOLHASSAN (I.C No: 830416-13-5439) Registration/Matric No: SHC080030 Name of Degree: DOCTOR OF PHILOSOPHY Title of Project Paper/Research Report/Disertation/Thesis (“this Work”): DIVERSITY OF POLYPORALES IN THE MALAY PENINSULAR AND THE APPLICATION OF GANODERMA AUSTRALE (FR.) PAT. IN BIOPULPING OF EMPTY FRUIT BUNCHES OF ELAEIS GUINEENSIS. Field of Study: MUSHROOM DIVERSITY AND BIOTECHNOLOGY I do solemnly and sincerely declare that: 1) I am the sole author/writer of this work; 2) This Work is original; 3) Any use of any work in which copyright exists was done by way of fair dealing and for permitted purposes and any excerpt or extract from, or reference to or reproduction of any copyright work has been disclosed expressly and sufficiently and the title of the Work and its authorship have been acknowledge in this Work; 4) I do not have any actual -

Jamur Polyporales Di Twa Suranadi Lombok Barat
Biopendix, Volume 7, Nomor 1, Desember 2020, hlm. 49-53 JAMUR POLYPORALES DI TWA SURANADI LOMBOK BARAT Meilinda Pahriana Sulastri*1, Hasan Basri2 Program Studi Biologi, Universitas Islam Al-Azhar, Mataram, NTB Coresponding author: Meilinda Pahriana Sulastri; Email: [email protected] Abstract Background: Mushrooms are a member of the kingdom of fungi, which has a high level of diversity in Indonesia. In general, fungi will grow in humid environmental conditions, especially during the rainy season on weathered wood, litter and trees. Macro fungi are fungi that form fruiting bodies and can be observed without using a microscope. Macro fungi generally consist of members of the Ascomycota and Basidomycota groups. This study aims to determine which mushrooms are included in the Polyporales order that grows in TWA Suranadi Methods: This research is descriptive exploratory by exploring and describing the macrophages found in TWA Suranadi. Sampling was carried out by the cruise method (Cruise Method). Identification is done by matching observational data in the form of morphological characteristics and environmental conditions using reference books and various scientific journals on macrofungal species. Results: The results showed that there were 7 species from 2 fungal families of the Polyporales order, namely: Trametes sp., Microporus sp. 1, Microporus sp. 2, Polyporus sp. 1, Polyporus sp. 2, Datronia sp. and Ganoderma sp. Conclusion: Based on the identification results, 2 families and 8 species were found belonging to the Polyporales order in TWA Suranadi, namely Trametes sp., Microporus sp. 1, Microporus sp. 2, Polyporus sp. 1, Polyporus sp. 2, Datronia sp., and Ganoderma sp. Keywords: fungi, Polyporales, TWA Suranadi Abstrak Latar Belakang: Jamur termasuk dalam anggota kingdom fungi yang memiliki tingkat keanekaragaman tinggi di Indonesia. -

(63) Continuation Inspart of Application No. PCT RE"SE SEN"I", "ES"E"NE
US 2010.0086647A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0086647 A1 Kristiansen (43) Pub. Date: Apr. 8, 2010 (54) FEED OR FOOD PRODUCTS COMPRISING filed on Jan. 25, 2006, provisional application No. FUNGALMATERAL 60/690,496, filed on Jun. 15, 2005. (75) Inventor: Bjorn Kristiansen, Frederikstad (30) Foreign Application Priority Data (NO) May 13, 2005 (DK) ........................... PA 2005 00710 Correspondence Address: Jun. 15, 2005 (DK). ... PA 2005 OO88O BROWDY AND NEIMARK, P.L.L.C. Jul. 15, 2005 (DK) ....................... PCTFDKO5/OO498 624 NINTH STREET, NW Jan. 25, 2006 (DK)........................... PA 2006 OO117 SUTE 300 WASHINGTON, DC 20001-5303 (US) Publication Classification 51) Int. Cl. (73)73) AssigneeA : MEDMUSHAS(DK) s HORSHOLM ( A2.3L I/28 (2006.01) A23K L/18 (2006.01) (21) Appl. No.: 11/914,318 A23K L/6 (2006.01) CI2P 19/04 (2006.01) (22) PCT Filed: May 11, 2006 AOIK 6L/00 (2006.01) (86). PCT NO. PCT/DKO6/OO2S3 (52) U.S. Cl. ................................ 426/62: 426/2: 119/230 S371 (c)(1) (57) ABSTRACT (2), (4) Date: Dec. 1, 2009 The present invention relates to feed and food compositions comprising material obtained by fermenting fungi of the Related U.S. Application Data Basidiomycetes family in a liquid medium. Interestingly, (63) DK2005/000498,continuation inspart filed onof Jul.application 15, 2005. No. PCT enhanceRE"SE Survival SEN"I",and/or support "ES"E"NE growth of normal, healthy (60) Provisional application No. 60/690,496, filed on Jun. animals. Furthermore, the compounds may modulate the 15, 2005, provisional application No. -

Phylogenetic Classification of Trametes
TAXON 60 (6) • December 2011: 1567–1583 Justo & Hibbett • Phylogenetic classification of Trametes SYSTEMATICS AND PHYLOGENY Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset Alfredo Justo & David S. Hibbett Clark University, Biology Department, 950 Main St., Worcester, Massachusetts 01610, U.S.A. Author for correspondence: Alfredo Justo, [email protected] Abstract: The phylogeny of Trametes and related genera was studied using molecular data from ribosomal markers (nLSU, ITS) and protein-coding genes (RPB1, RPB2, TEF1-alpha) and consequences for the taxonomy and nomenclature of this group were considered. Separate datasets with rDNA data only, single datasets for each of the protein-coding genes, and a combined five-marker dataset were analyzed. Molecular analyses recover a strongly supported trametoid clade that includes most of Trametes species (including the type T. suaveolens, the T. versicolor group, and mainly tropical species such as T. maxima and T. cubensis) together with species of Lenzites and Pycnoporus and Coriolopsis polyzona. Our data confirm the positions of Trametes cervina (= Trametopsis cervina) in the phlebioid clade and of Trametes trogii (= Coriolopsis trogii) outside the trametoid clade, closely related to Coriolopsis gallica. The genus Coriolopsis, as currently defined, is polyphyletic, with the type species as part of the trametoid clade and at least two additional lineages occurring in the core polyporoid clade. In view of these results the use of a single generic name (Trametes) for the trametoid clade is considered to be the best taxonomic and nomenclatural option as the morphological concept of Trametes would remain almost unchanged, few new nomenclatural combinations would be necessary, and the classification of additional species (i.e., not yet described and/or sampled for mo- lecular data) in Trametes based on morphological characters alone will still be possible. -

Hymenochaetaceae from Paraguay: Revision of the Family and New Records
Current Research in Environmental & Applied Mycology (Journal of Fungal Biology) 10(1): 242–261 (2020) ISSN 2229-2225 www.creamjournal.org Article Doi 10.5943/cream/10/1/24 Hymenochaetaceae from Paraguay: revision of the family and new records Maubet Y1, Campi M1* and Robledo G2,3,4 1Universidad Nacional de Asunción. Laboratorio de Análisis de Recursos Vegetales Área Micología-Facultad de Ciencias Exactas y Naturales 2BioTecA3 – Centro de Biotecnología Aplicada al Agro y Alimentos, Facultad de Ciencias Agropecuarias – Univ. Nac. de Córdoba, Ing. Agr. Félix Aldo Marrone 746 – Planta Baja CC509 – CP 5000, Ciudad Universitaria, Córdoba, Argentina 3CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina 4Fundación Fungicosmos, www.fungicosmos.org, Córdoba, Argentina Maubet Y, Campi M, Robledo G 2020 – Hymenochaetaceae from Paraguay: revision of the family and new records. Current Research in Environmental & Applied Mycology (Journal of Fungal Biology) 10(1), 242–261, Doi 10.5943/cream/10/1/24 Abstract A synopsis of species of Hymenochaetaceae from five departments of Paraguay (Alto Paraguay, Boquerón, Central, Cordillera and Paraguarí) is presented. Thirteen species from nine genera are reported, of which eleven are recorded for the first time. Descriptions and macro- and microscopic illustrations are presented for each species. Discussions on their taxonomy and ecology are provided. Key words – fungal diversity – Hymenochaetales – neotropical polypores – taxonomy Introduction Hymenochaetaceae was proposed by Donk (1948) and is characterized by the permanent xantochroic reaction (a dark coloration in alkali), the lack of clamp connections and the presence of setae in some species (Donk 1948, Hibbett et al. 2014, Ryvarden 2004). Most of the species of this family were traditionally placed among two main genera: Phellinus s.l. -

A Phylogenetic Overview of the Antrodia Clade (Basidiomycota, Polyporales)
Mycologia, 105(6), 2013, pp. 1391–1411. DOI: 10.3852/13-051 # 2013 by The Mycological Society of America, Lawrence, KS 66044-8897 A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales) Beatriz Ortiz-Santana1 phylogenetic studies also have recognized the genera Daniel L. Lindner Amylocystis, Dacryobolus, Melanoporia, Pycnoporellus, US Forest Service, Northern Research Station, Center for Sarcoporia and Wolfiporia as part of the antrodia clade Forest Mycology Research, One Gifford Pinchot Drive, (SY Kim and Jung 2000, 2001; Binder and Hibbett Madison, Wisconsin 53726 2002; Hibbett and Binder 2002; SY Kim et al. 2003; Otto Miettinen Binder et al. 2005), while the genera Antrodia, Botanical Museum, University of Helsinki, PO Box 7, Daedalea, Fomitopsis, Laetiporus and Sparassis have 00014, Helsinki, Finland received attention in regard to species delimitation (SY Kim et al. 2001, 2003; KM Kim et al. 2005, 2007; Alfredo Justo Desjardin et al. 2004; Wang et al. 2004; Wu et al. 2004; David S. Hibbett Dai et al. 2006; Blanco-Dios et al. 2006; Chiu 2007; Clark University, Biology Department, 950 Main Street, Worcester, Massachusetts 01610 Lindner and Banik 2008; Yu et al. 2010; Banik et al. 2010, 2012; Garcia-Sandoval et al. 2011; Lindner et al. 2011; Rajchenberg et al. 2011; Zhou and Wei 2012; Abstract: Phylogenetic relationships among mem- Bernicchia et al. 2012; Spirin et al. 2012, 2013). These bers of the antrodia clade were investigated with studies also established that some of the genera are molecular data from two nuclear ribosomal DNA not monophyletic and several modifications have regions, LSU and ITS. A total of 123 species been proposed: the segregation of Antrodia s.l. -

A Re-Evaluation of Neotropical Junghuhnia S.Lat. (Polyporales, Basidiomycota) Based on Morphological and Multigene Analyses
Persoonia 41, 2018: 130–141 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2018.41.07 A re-evaluation of Neotropical Junghuhnia s.lat. (Polyporales, Basidiomycota) based on morphological and multigene analyses M.C. Westphalen1,*, M. Rajchenberg2, M. Tomšovský3, A.M. Gugliotta1 Key words Abstract Junghuhnia is a genus of polypores traditionally characterised by a dimitic hyphal system with clamped generative hyphae and presence of encrusted skeletocystidia. However, recent molecular studies revealed that Mycodiversity Junghuhnia is polyphyletic and most of the species cluster with Steccherinum, a morphologically similar genus phylogeny separated only by a hydnoid hymenophore. In the Neotropics, very little is known about the evolutionary relation- Steccherinaceae ships of Junghuhnia s.lat. taxa and very few species have been included in molecular studies. In order to test the taxonomy proper phylogenetic placement of Neotropical species of this group, morphological and molecular analyses were carried out. Specimens were collected in Brazil and used for DNA sequence analyses of the internal transcribed spacer and the large subunit of the nuclear ribosomal RNA gene, the translation elongation factor 1-α gene, and the second largest subunit of RNA polymerase II gene. Herbarium collections, including type specimens, were studied for morphological comparison and to confirm the identity of collections. The molecular data obtained revealed that the studied species are placed in three different genera. Specimens of Junghuhnia carneola represent two distinct species that group in a lineage within the phlebioid clade, separated from Junghuhnia and Steccherinum, which belong to the residual polyporoid clade. -

(12) United States Patent (10) Patent No.: US 8,765,138 B2 Stamets (45) Date of Patent: Jul
US008765138B2 (12) United States Patent (10) Patent No.: US 8,765,138 B2 Stamets (45) Date of Patent: Jul. 1, 2014 (54) ANTIVIRAL AND ANTIBACTERIAL Moore et al. "Fungal Products as Food” pp. 1-17. Reprint of Moore, ACTIVITY FROMIMEDICINAL D. & Chiu, S. W. Fungal products as food. Chapter 10 in Bio MUSHROOMS Exploitation of Fkilamentous Fungi (ed S.B. Pointing & K. D. Hyde), pp. 223-251. Fungal Diversity Press: Hong Kong.* (76) Inventor: Paul Edward Stamets, Shelton, WA “Tinctura Cinchonae Compsita' from King's American Dispensa (US) tory, 1898. Retrieved from: Henriette's Herbal Homepage on May 2, 2012. Retrieved from: <URL: http://www.henriettesherbal.com/ (*) Notice: Subject to any disclaimer, the term of this eclectic/kings, cinchona tinc 1.html>. patent is extended or adjusted under 35 Schar, D. Plant botanic. Retrieved from the Interneton: May 2, 2012. U.S.C. 154(b) by 1183 days. Retrieved from: <URL: http://www.planetbotanic.ca/maitake jour nal materia.htm>. (21) Appl. No.: 12/284,646 "Alcohol.-Alcohol, U.S.P. From: A Handbook of Useful Drugs, by State Medical Examining and Licensing Boards, Press of the Ameri (22) Filed: Sep. 24, 2008 can Medical Association: 1913.Retrieved from the Internet on: May 2, 2012. Retrieved from the Internet: <URL: http://chestofbooks. (65) Prior Publication Data com/health/materia-medica-drugs/American-Medical-Association? A-Handbook-of-Useful-Drugs).* US 2009/O 130 138A1 May 21, 2009 Hobbs, C. Medicinal Mushrooms (Herbs and Health Series). Feb. 1. 2002. Retrieved from the Internet on: May 2, 2012. Retrieved from: Related U.S. -

Wood-Rotting Fungi in East Khasi Hills of Meghalaya, Northeast India, with Special Reference to Heterobasidion Perplexa (A Rare Species ‒ New to India)
Current Research in Environmental & Applied Mycology 4 (1): 117–124 (2014) ISSN 2229-2225 www.creamjournal.org Article CREAM Copyright © 2014 Online Edition Doi 10.5943/cream/4/1/10 Wood-rotting fungi in East Khasi Hills of Meghalaya, northeast India, with special reference to Heterobasidion perplexa (a rare species ‒ new to India) Lyngdoh A1,2* and Dkhar MS1 1Microbial Ecology Laboratory, Department of Botany, North Eastern Hill University, Shillong- 793022, Meghalaya, India. 2Department of Botany, Shillong College, Shillong – 793003, Meghalaya, India. Email: [email protected] Lyngdoh A, Dkhar MS 2014 ‒ Wood-rotting fungi in East Khasi Hills of Meghalaya, Northeast India, with special reference to Heterobasidion perplexa (a rare species ‒ new to India). Current Research in Environmental & Applied Mycology 4 (1): 117–124, Doi 10.5943/cream/4/1/10 Abstract Field surveys and collection of the basidiocarps of wood-rotting fungi were carried out in eight forest stands of East Khasi Hills district of Meghalaya, India. Seventy eight wood-rotting fungi belonging to 23 families were identified. The undisturbed Mawphlang sacred grove was found to harbour a much larger number of the wood-rotting fungi (33.54 %) as compared to the other forest stands studied. Similarly, logs also harboured the maximum number of wood-rotting fungi (59.7 %) while living trees harboured the least (7.8%). Microporus xanthopus had the highest frequency percentage of occurrence with 87.5 %, followed by Cyclomyces tabacinus, Microporus affinis and Trametes versicolor with 62.5 %. Majority of the wood-rotting fungi are white-rot fungi (89.61%) and only few are brown-rots. -

Short Title: Lentinus, Polyporellus, Neofavolus
In Press at Mycologia, preliminary version published on February 6, 2015 as doi:10.3852/14-084 Short title: Lentinus, Polyporellus, Neofavolus Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa Jaya Seelan Sathiya Seelan Biology Department, Clark University, 950 Main Street, Worcester, Massachusetts 01610, and Institute for Tropical Biology and Conservation (ITBC), Universiti Malaysia Sabah, 88400 Kota Kinabalu, Sabah, Malaysia Alfredo Justo Laszlo G. Nagy Biology Department, Clark University, 950 Main Street, Worcester, Massachusetts 01610 Edward A. Grand Mahidol University International College (Science Division), 999 Phuttamonthon, Sai 4, Salaya, Nakorn Pathom 73170, Thailand Scott A. Redhead ECORC, Science & Technology Branch, Agriculture & Agri-Food Canada, CEF, Neatby Building, Ottawa, Ontario, K1A 0C6 Canada David Hibbett1 Biology Department, Clark University, 950 Main Street Worcester, Massachusetts 01610 Abstract: The genus Lentinus (Polyporaceae, Basidiomycota) is widely documented from tropical and temperate forests and is taxonomically controversial. Here we studied the relationships between Lentinus subg. Lentinus sensu Pegler (i.e. sections Lentinus, Tigrini, Dicholamellatae, Rigidi, Lentodiellum and Pleuroti and polypores that share similar morphological characters). We generated sequences of internal transcribed spacers (ITS) and Copyright 2015 by The Mycological Society of America. partial 28S regions of nuc rDNA and genes encoding the largest subunit of RNA polymerase II (RPB1), focusing on Lentinus subg. Lentinus sensu Pegler and the Neofavolus group, combined these data with sequences from GenBank (including RPB2 gene sequences) and performed phylogenetic analyses with maximum likelihood and Bayesian methods. We also evaluated the transition in hymenophore morphology between Lentinus, Neofavolus and related polypores with ancestral state reconstruction. -

Piptoporus Betulinus, Fomitopsis Betulina)
Birch Fungi – Razor Strop, Birch Bracket (Piptoporus betulinus, Fomitopsis betulina) Features - This distinctive fungus only grows on birches, looks like nothing else that grows on birches, and is very common. It is not an aggressive tree killer, but is instead primarily in the business of decomposing dead trees. Birch polypore is present throughout the range of the birches, which grow around the globe in the northern hemisphere. The white-to-brownish fruiting bodies are annual, emerging from the bark of birches in spring and summer, but they deteriorate slowly and are still visible through the winter, though by then they have blackened and are not so attractive. Global Uses – Otzi the Iceman, who lived 5300 years ago, carried two fragments of a fruiting body of Fomitopsis betulina (formerly Piptoporus betulinus). Some scientists believe that Ӧtzi might have used the fungus for medical purposes and, although the idea arouses some controversy, the long tradition of the use of F. betulina in folk medicine is a fact. Infusion from F. betulina fruiting bodies was popular, especially in Russia, Baltic countries, Hungary, Romania for its nutritional and calming properties. Fungal tea was used against various cancer types, as an immunoenhancing, anti-parasitic agent, and a remedy for gastrointestinal disorders. Antiseptic and anti-bleeding dressings made from fresh F. betulina fruiting body were applied to wounds and the powder obtained from dried ones was used as a painkiller. Was used as a razor strop to sharpen fine edged blades. Medicinal Potential – Chemical Constituents: A and C, 1,3-beta-D-glucopyranan, B ergosta-7,22-dien-3- ol, fungisterol, ergosterol, agaric acid, dehydrotumulosic acid, ungalinic acid, betulinic acid, and tumulosic acid.