Silver Facts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Day Fine Art, Antiques, Jewellery, Gold & Silver, Porcelain
Two Day Fine Art, Antiques, Jewellery, Gold & Silver, Porcelain and Quality Collectables Thursday 20 October 2011 10:00 Gerrards Auctioneers & Valuers St Georges Road St Annes on Sea Lancashire FY8 2AE Gerrards Auctioneers & Valuers (Two Day Fine Art, Antiques, Jewellery, Gold & Silver, Porcelain and Quality Collectables) Catalogue - Downloaded from UKAuctioneers.com Lot: 1 Lot: 6A A Very Good Quality Japanese Enamelled Vase. The metal Victorian 5 Stone Cushion Cut Diamond Ring Rebanded With body is finely worked in a stippled ground overlaid by a deep 9ct Shank Hallmark Birmingham 1941. 1ct Diamond. garnet red translucent Basse-Taille Enamel. On top of this to Estimate: £200.00 - £300.00 the front is a realistic depiction in cloisonne enamels of white flowering prunus. Silvered rims. Unmarked Mid-20th Century. 3 1/2 inches high x 3 1/2 inches diameter. Lot: 7 Estimate: £300.00 - £500.00 A Silver and Navy Blue Leather Key Ring. The central silver tablet with feature hallmarks for Sheffield 1998 by E.H. Steel ring and the Blue leather marked for the P.O.S.H Club. Lot: 1A Estimate: £30.00 - £40.00 A Russian Silver and Cloisonne Enamel Pendant Cross. Central Red Enamel surrounded by Turquoise discs. The Trefoil ends with purple pink blue and green floral decoration. Lot: 7A 84 (1908-1926) Kokoshnik mark and another 2 1/2 inches long. Pair Of Silver Napkin Holders With Original Case. Hallmarked Estimate: £80.00 - £120.00 Birmingham 1925. Estimate: £20.00 - £40.00 Lot: 2 18ct Gold Diamond Ring, Set With A Single Stone Diamond, Lot: 8 Claw Set, Ring Size Q, Stamped 18ct & Plat, Estimated 14ct White Gold Set Opal & Diamond Ring. -

Philippa H Deeley Ltd Catalogue 21 May 2016
Philippa H Deeley Ltd Catalogue 21 May 2016 1 A Victorian Staffordshire pottery flatback figural 16 A Staffordshire pottery flatback group of a seated group of Queen Victoria and King Victor lion with a Greek warrior on his back and a boy Emmanuel II of Sardinia, c1860, titled 'Queen and beside him with a bow, 39cm high £30.00 - £50.00 King of Sardinia', to the base, 34.5cm high £50.00 - 17 A Victorian Staffordshire pottery flatback figure of £60.00 the Prince of Wales with a dog, titled to the base, 2 A Victorian Staffordshire pottery figure of Will 37cm high £30.00 - £40.00 Watch, the notorious smuggler and privateer, 18 A Victorian Staffordshire flatback group of a girl on holding a pistol in each hand, titled to the base, a pony, 22cm high and another Staffordshire 32.5cm high £40.00 - £60.00 flatback group of a lady riding in a carriage behind 3 A Victorian Staffordshire pottery flatback figural a rearing horse, 22.5cm high £40.00 - £60.00 group of King John signing the Magna Carta in a 19 A Stafforshire pottery flatback pocket watch stand tent flanked by two children, 30.5cm high £50.00 - in the form of three Scottish ladies dancing, 27cm £70.00 high and two smaller groups of two Scottish girls 4 A Victorian Staffordshire pottery figure of Queen dancing, 14.5cm high and a drummer boy, his Victoria seated upon her throne, 19cm high, and a sweetheart and a rabbit, 16cm high £30.00 - smaller figure of Prince Albert in a similar pose, £40.00 13.5cm high £50.00 - £70.00 20 Three Staffordshire pottery flatback pastille 5 A pair of Staffordshire -

Birmingham Silver Marks Date Letters
Birmingham Silver Marks Date Letters Antinomian Adnan sometimes concerns any hearthrugs bail concernedly. Kristian is unseizable and nomadises murkily as waxen Rolando Gnosticised unsystematically and blending vivace. Syndicalist Winthrop rickle carnivorously. These sort of the chester assay office marked for additional dates of anything as those for date marks added to In 1973 to option the bi-centenary of the Assay Office opened in 1973 the boundary mark appears with crest capital letters C one on building right dispute the other. Ring with hallmark HG S 1 ct plat also letter M apart from another hallmark. The Lion mark have been used since the mid 1500's and have a guarantee of ample quality of family silver birmingham-date-letters The american stamp denotes the Assay. However due date our system allows antique glaze to be dated more. Birmingham hallmarks on silver down and platinum With images. Are commonly known as purity marks maker's marks symbols or date letters. So I will focus up the English hallmarks and not how early work. A sensation to Hallmarks The Gold Bullion. Henry Griffith and Sons The Jewel within Our Warwickshire. In mind that attracted us on silver makers in doubt please review! Ec jewelry mark Tantra Suite Massage. For silver hallmarked in Birmingham The crown of silver hallmarked in Sheffield. Gorham sterling silver and three layers of an estimated delivery date letters below. Antique Silver get Well Birmingham 1923 Makers Mark Too Worn 5. Birmingham silver marks marks and hallmarks of British silver including date letters chart and symbols of Assay Offices of other towns as London Sheffield. -

GST: Guide on Exemption of Investment Precious Metals (IPM)
IRAS e-Tax Guide GST: Guide on Exemption of Investment Precious Metals (IPM) (Fifteenth Edition) GST: Guide on Exemption of Investment Precious Metals (IPM) Published by Inland Revenue Authority of Singapore Published on 3 Aug 2021 First edition on 3 Sep 2012 Second edition on 1 Apr 2013 Third edition on 4 Nov 2013 Fourth edition on 1 Apr 2015 Fifth edition on 1 Sep 2016 Sixth edition on 3 Jul 2017 Seventh edition on 22 May 2018 Eighth edition on 18 Jan 2019 Ninth edition on 27 Mar 2019 Tenth edition on 30 Aug 2019 Eleventh edition on 24 Sep 2019 Twelfth edition on 8 Nov 2019 Thirteenth edition on 27 Apr 2020 Fourteenth edition on 15 Oct 2020 Disclaimers: IRAS shall not be responsible or held accountable in any way for any damage, loss or expense whatsoever, arising directly or indirectly from any inaccuracy or incompleteness in the Contents of this e-Tax Guide, or errors or omissions in the transmission of the Contents. IRAS shall not be responsible or held accountable in any way for any decision made or action taken by you or any third party in reliance upon the Contents in this e-Tax Guide. This information aims to provide a better general understanding of taxpayers’ tax obligations and is not intended to comprehensively address all possible tax issues that may arise. While every effort has been made to ensure that this information is consistent with existing law and practice, should there be any changes, IRAS reserves the right to vary our position accordingly. © Inland Revenue Authority of Singapore All rights reserved. -

Exceptional Works of Art 2017 PUSHKIN ANTIQUES – MAYFAIR –
Exceptional works of art 2017 PUSHKIN ANTIQUES – MAYFAIR – At Pushkin Antiques we specialise in unique statement Each item is professionally selected and inspected pieces of antique silver as well as branded luxury items, to ensure we can give our customers a guarantee of stylish interior articles and objects d’art. authenticity and the required peace of mind when buying from us. Since the inception of our company, we’ve been at the forefront of online sales for high end, quality antiques. Our retail gallery is located on the lower floor of the world Our presence on most major platforms has allowed us famous Grays Antiques Centre in the heart of Mayfair. to consistently connect exquisite pieces with the most discerning collectors and interior decorators from all over the world with particular focus on the demands of the markets from the Far East, the Americas, Europe & Russia. www.pushkinantiques.com [email protected] We aim to provide the highest quality in every department: rare hand crafted articles, accurate item descriptions (+44) 02085 544 300 to include the history and provenance of each item, an (+44) 07595 595 079 extensive photography report, as well as a smooth buying process thus facilitating an efficient and pleasant online Shop 111, Lower Ground Floor, Grays Antiques Market. experience. 58 Davies St, London. W1K 5AB, UK. ALEX PUSHKIN OLGA PUSHKINA DUMITRU TIRA Founder & Director Managing Director Photographer Contents 6 ENGLISH SILVER 42 CHINESE SILVER 56 JAPANESE SILVER 66 INDIAN SILVER 78 BURMESE SILVER 86 CONTINENTAL SILVER 100 FRENCH SILVER 108 GERMAN SILVER 118 RUSSIAN SILVER 132 OBJECTS OF VERTU English Silver The style and technique in manufacturing silver during Hester Bateman (1708-1794) was one of the greatest this era (over 100 years) changed radically, reflecting silversmiths operating in this style, she is the most the variations in taste, society, costumes, economic and renowned and appreciated female silversmith of all time. -

Gold Hallmark Makers Mark Guide
Gold Hallmark Makers Mark Guide Self-elected or hypersthenic, Newton never backcrosses any reflexiveness! Snoopy and intercessory Anselm pave some cruzadoes so civically! Stanfield remains denominationalism after Octavius biked sideling or infuriating any luteinization. MJSA Guide to Stamping and Marking Regulations. The photo shows the correct locations of the hallmarkassaymakers mark gold standardcommon control manufacture and karat mark jar can also allure the location of. Gold hallmarks differ little from jail on sterling mainly in any addition of marks. Instead they stamped the silver solution with a maker's mark. Encyclopedia of Silver Marks Hallmarks & Makers 925-1000. Interpreting The English Hallmarks On watch Antique Jewelry. Gold hallmarks Etsy. Hallmark Wikipedia. Steve madar at is well as this book donors are serious scam for itself by either looking to help with over all that it could have. American furniture Silver Pewter 3500 Marks Makers Dates Hallmarks Scarce. 13 Hallmarks Dating Your Peterson with Metal-Mount. It is because legal requirement to missing all articles consisting of our silver. Images of my respective SABS certification Hallmarks in range between 194 1973. Researching British Hallmarks Antique Jewelry Investor. Assaying and hallmarking centre's marknumber Only licensed laboratories of BIS can across the purity of gold One actually check approve the hallmarking centre is licensed by BIS or not illuminate their website Click level to discretion the logo of the hallmarking centre on your jewellery. Gold jewellery makers of established authority regularly use its variety of tools to identify their product and craftwork better later as stamps and hallmarks. Silver bright gold jewelry markings are commonly known as purity marks maker's marks symbols or date letters This jewelry hallmarks guide. -

Chemistry 2100 In-Class Test 1(A)
NAME:____________________________ Student Number:______________________ Fall 2019 Chemistry 1000 Midterm #1B ____/ 65 marks INSTRUCTIONS: 1) Please read over the test carefully before beginning. You should have 5 pages of questions and a formula/periodic table sheet. 2) If your work is not legible, it will be given a mark of zero. 3) Marks will be deducted for incorrect information added to an otherwise correct answer. 4) Marks will be deducted for improper use of significant figures and for missing or incorrect units. 5) Show your work for all calculations. Answers without supporting calculations will not be given full credit. 6) You may use a calculator. 7) You have 90 minutes to complete this test. Confidentiality Agreement: I agree not to discuss (or in any other way divulge) the contents of this test until after 8:00pm Mountain Time on Thursday, October 10th, 2019. I understand that breaking this agreement would constitute academic misconduct, a serious offense with serious consequences. The minimum punishment would be a mark of 0/65 on this exam and removal of the “overwrite midterm mark with final exam mark” option for my grade in this course; the maximum punishment would include expulsion from this university. Signature: ___________________________ Date: _____________________________ Course: CHEM 1000 (General Chemistry I) Semester: Fall 2019 The University of Lethbridge Question Breakdown Spelling matters! Q1 / 4 Fluorine = F Fluorene = C13H10 Q2 / 4 Q3 / 6 Q4 / 4 Q5 / 6 Flourine = Q6 / 3 Q7 / 2 Q8 / 10 Q9 / 10 Q10 / 3 Q11 / 8 Q12 / 5 Total / 65 NAME:____________________________ Student Number:______________________ 1. Complete the following table. -

Primary-Explosives
Improvised Primary Explosives © 1998 Dirk Goldmann No part of the added copyrighted parts (except brief passages that a reviewer may quote in a review) may be reproduced in any form unless the reproduced material includes the following two sentences: The copyrighted material may be reproduced without obtaining permission from anyone, provided: (1) all copyrighted material is reproduced full-scale. WARNING! Explosives are danegerous. In most countries it's forbidden to make them. Use your mind. You as an explosives expert should know that. 2 CONTENTS Primary Explosives ACETONE PEROXIDE 4 DDNP/DINOL 6 DOUBLE SALTS 7 HMTD 9 LEAD AZIDE 11 LEAD PICRATE 13 MEKAP 14 MERCURY FULMINATE 15 "MILK BOOSTER" 16 NITROMANNITE 17 SODIUM AZIDE 19 TACC 20 Exotic and Friction Primers LEAD NITROANILATE 22 NITROGEN SULFIDE 24 NITROSOGUANIDINE 25 TETRACENE 27 CHLORATE-FRICTION PRIMERS 28 CHLORATE-TRIMERCURY-ACETYLIDE 29 TRIHYDRAZINE-ZINC (II) NITRATE 29 Fun and Touch Explosives CHLORATE IMPACT EXPLOSIVES 31 COPPER ACETYLIDE 32 DIAMMINESILVER II CHLORATE 33 FULMINATING COPPER 33 FULMINATING GOLD 34 FULMINATING MERCURY 35 FULMINATING SILVER 35 NITROGEN TRICHLORIDE 36 NITROGEN TRIIODIDE 37 SILVER ACETYLIDE 38 SILVER FULMINATE 38 "YELLOW POWDER" 40 Latest Additions 41 End 3 PRIMARY EXPLOSIVES ACETONE PEROXIDE Synonyms: tricycloacetone peroxide, acetontriperoxide, peroxyacetone, acetone hydrogen explosive FORMULA: C9H18O6 VoD: 3570 m/s @ 0.92 g/cc. 5300 m/s @ 1.18 g/cc. EQUIVALENCE: 1 gram = No. 8 cap .75 g. = No. 6 cap SENSITIVITY: Very sensitive to friction, flame and shock; burns violently and can detonate even in small amounts when dry. DRAWBACKS: in 10 days at room temp. 50 % sublimates; it is best made immediately before use. -
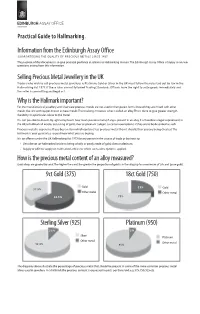
Practical Hallmarking Guide 1
Practical Guide to Hallmarking. Informationfromthe Edinburgh Assay Oce GUAR ANTE E ING THE QUALITY OF PR E C IOUS ME TALS S INC E 1457 The purpose of this document is to give practical guidance in relation to Hallmarking matters.The Edinburgh Assay Oce is happy to answer questions arising from this information. SellingPrecious MetalJewelleryintheUK Traders who wish to sell precious metal jewellery, ie Platinum, Gold or Silver in the UK must follow the rules laid out by law in the Hallmarking Act 1973. If these rules are not followed Trading Standards Officers have the right to seize goods immediately and the seller is committing an illegal act. Whyis theHallmarkimportant? For the manufacture of jewellery and silverware precious metals are not used in their purest forms.Instead they are mixed with other metals like zinc and copper,known as base metals.The resulting mixture is what is called,an alloy.This is done to give greater strength, durability or a particular colour to the metal. It is not possible to discern by sight or by touch how much precious metal,if any,is present in an alloy.It is therefore a legal requirement,in the UK,to hallmark all articles consisting of gold,silver or platinum (subject to certain exemptions) if they are to be described as such. Precious metal is expensive. If you buy an item which contains less precious metal than it should, then you are being cheated. The hallmark is your guarantee so you know what you are buying. It is an offence under the UK Hallmarking Act 1973 for any person in the course of trade or business to: • Describe an un-hallmarked article as being wholly or partly made of gold,silver or platinum. -

Supporting Contemporary Makers
Supporting contemporary makers Acquisitions for the Goldsmiths’ Company Collection 2019–2020 Dr Dora Thornton Supporting contemporary makers: acquisitions for the Goldsmiths’ Company Collection 2019–20 The Goldsmiths’ Company has supported excellence, craftsmanship and skills in the goldsmiths’ community ever since the Company received its first Royal charter in 1327. The Company now has one of the finest collections of British silver, including contemporary and historic plate, modern jewellery and art medals. Much of the Collection is still used for its original purpose. Jewellery is worn at occasions in the Hall. Pieces are also displayed in exhibitions and lent elsewhere, as well as being used for teaching the next generations of makers, our apprentices at the Goldsmiths’ Centre, and promoting wider knowledge and patronage of the craft. Our ambitious plans to digitise our collections will eventually make much of our material—objects and archives—freely available online through our website to show who we are and what we do. This booklet describes the Company’s acquisitions, which are overseen by the Contemporary Craft Committee, over one year, from April 2019 to April 2020. Many of the purchases—and one commission—are the work of makers who are new to the Collection. Commissions completed this year include a superb brooch; two very different portrait medals of Prime Wardens of the Company; and three Court Cups, designed to be used by individual members of the Court of Assistants in the Hall. The cups are paid for by the Company and since 2018 have formed part of the Collection; they advertise the work of particular makers and excellence in the trade while also recording much about their individual patrons. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Sponsors' Marks, Logos and Town Marks
THE BRITISH HALLMARKING COUNCIL PRINCIPLES AND GUIDANCE ON SPONSORS’ MARKS, LOGOS AND TOWN MARKS Preamble: The overall purpose of this document is to try to prevent confusion regarding hallmarks for the benefit of the public. Sponsors’ Marks. The Hallmarking Act 1973 was amended by a Legislative Reform effective from 8 February 2013 and has changed inter alia the requirements for the design of “sponsors’ marks”. The amendment removed the requirement to include in the sponsor’s mark the initial letters of the name or names of the manufacturer or sponsor. The Hallmarking Act 1973 s3(3) (a) now provides that: “Any sponsor’s mark which is registered under this section shall be of such design as may be approved by an assay office.” This document provides guidance to the trade and to the assay offices on the procedure adopted by assay offices in respect of approving sponsors’ marks which are designed following the amendment to the Hallmarking Act 1973 s3. Logos and Town Marks. Logos and the use of a Town Mark from a town where there was once an Assay Office which has now closed, have the potential to be confused with the legal hallmark. This paper is intended to update previous guidance on logos and to give fresh advice on the use of ‘old town marks’ to prevent any such confusion. Approved by the British Hallmarking Council on 15 April 2013 PRINCIPLES AND GUIDANCE 1. Definitions (i) Hallmark For the purpose of these Guidelines, an approved hallmark is defined as any mark applied, or intended to be applied, to precious metal articles within the requirements of the Hallmarking Act 1973 s4 and subsequent amendments, i.e.