Kogia Breviceps) and Dwarf (K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of Southern Ocean Squids Using Nets and Beaks
Marine Biodiversity (2020) 50:98 https://doi.org/10.1007/s12526-020-01113-4 REVIEW A review of Southern Ocean squids using nets and beaks Yves Cherel1 Received: 31 May 2020 /Revised: 31 August 2020 /Accepted: 3 September 2020 # Senckenberg Gesellschaft für Naturforschung 2020 Abstract This review presents an innovative approach to investigate the teuthofauna from the Southern Ocean by combining two com- plementary data sets, the literature on cephalopod taxonomy and biogeography, together with predator dietary investigations. Sixty squids were recorded south of the Subtropical Front, including one circumpolar Antarctic (Psychroteuthis glacialis Thiele, 1920), 13 circumpolar Southern Ocean, 20 circumpolar subantarctic, eight regional subantarctic, and 12 occasional subantarctic species. A critical evaluation removed five species from the list, and one species has an unknown taxonomic status. The 42 Southern Ocean squids belong to three large taxonomic units, bathyteuthoids (n = 1 species), myopsids (n =1),andoegopsids (n = 40). A high level of endemism (21 species, 50%, all oegopsids) characterizes the Southern Ocean teuthofauna. Seventeen families of oegopsids are represented, with three dominating families, onychoteuthids (seven species, five endemics), ommastrephids (six species, three endemics), and cranchiids (five species, three endemics). Recent improvements in beak identification and taxonomy allowed making new correspondence between beak and species names, such as Galiteuthis suhmi (Hoyle 1886), Liguriella podophtalma Issel, 1908, and the recently described Taonius notalia Evans, in prep. Gonatus phoebetriae beaks were synonymized with those of Gonatopsis octopedatus Sasaki, 1920, thus increasing significantly the number of records and detailing the circumpolar distribution of this rarely caught Southern Ocean squid. The review extends considerably the number of species, including endemics, recorded from the Southern Ocean, but it also highlights that the corresponding species to two well-described beaks (Moroteuthopsis sp. -

2017 377 Encyclopedia of Whales, Dolphins and Porpoises
2017 BOOK REVIEWS 377 Encyclopedia of Whales, Dolphins and Porpoises By Erich Hoyt. 2017. Firefly Books. 300 pages, 49.95 CAD, Cloth. Written by a British-based, dual-citizen Canadian tion that individual animals could be photographed and who is a research scientist, conservationist, and author, identified by distinctive species-specific features, such the Encyclopedia of Whales, Dolphins and Porpoises as flukes, dorsal fins, pigmentation patterns, scars, and provides an interesting and beautiful global overview wounds. this led to great advances in previously dif- of cetaceans. Part pictorial guide, part research over - ficult areas to research such as migration, distribution, view, part coffee table book, and part call to action, and social behaviour. In a general book such as this and brimming with incredibly beautiful photographs obviously not all biological facts can be provided, but showing cetaceans in action, this book will appeal to it does provide an interesting and sometimes astound- many readers in its attractive, easy-to-read format. ing array of biological information. It is quite enlight- the reader will learn a great deal. the book contains ening how little is still known about some cetacean many interesting facts about this hugely popular yet species, even breeding areas and species taxonomy, mystical group of marine mammals. In recounting the and how recently much of the known scientific infor- history of cetacean research and monitoring, the author mation has been gathered. It was sobering to learn that emphasized the major progress made with the realiza- almost half of all cetaceans globally are considered 378 THE CANADIAN FIELD -N ATURALIST Vol. -

Resolution 3.11 Conservation Plan for Black Sea Cetaceans
ACCOBAMS-MOP3/2007/Res.3.11 RESOLUTION 3.11 CONSERVATION PLAN FOR BLACK SEA CETACEANS The Meeting of the Parties to the Agreement on the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and contiguous Atlantic area: On the recommendation of the ACCOBAMS Scientific Committee, Aware that all three Black Sea cetacean species, the harbour porpoise (Phocoena phocoena), the short-beaked common dolphin (Delphinus delphis) and the common bottlenose dolphin (Turpsiops truncatus), experienced a dramatic decline in abundance during the twentieth century, Taking into account that the International Union for the Conservation of Nature (IUCN)-ACCOBAMS workshop on the Red List Assessment of Cetaceans in the ACCOBAMS Area (Monaco, March 2006) concluded that the Black Sea populations of the harbour porpoise, common dolphin and bottlenose dolphin are endangered, Conscious that most of the factors responsible for their decline, such as current fisheries by-catches, extensive habitat degradation and other anthropogenic impacts, pose continuous threats to the existence of cetaceans in the Black Sea and contiguous waters, represented by the Sea of Azov, the Kerch strait and the Turkish straits system (including the Bosphorus strait, the Marmara Sea and the Dardanelles straits), Convinced that the plan is an integral component of discussions on Black Sea regional and national strategies, plans, programmes and projects concerned with the protection, exploration and management of the Black Sea environment, biodiversity, living resources, marine mammals -

Is Harbor Porpoise (Phocoena Phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments?
animals Article Is Harbor Porpoise (Phocoena phocoena) Exhaled Breath Sampling Suitable for Hormonal Assessments? Anja Reckendorf 1,2 , Marion Schmicke 3 , Paulien Bunskoek 4, Kirstin Anderson Hansen 1,5, Mette Thybo 5, Christina Strube 2 and Ursula Siebert 1,* 1 Institute for Terrestrial and Aquatic Wildlife Research, University of Veterinary Medicine Hannover, Werftstrasse 6, 25761 Buesum, Germany; [email protected] (A.R.); [email protected] (K.A.H.) 2 Centre for Infection Medicine, Institute for Parasitology, University of Veterinary Medicine Hannover, Buenteweg 17, 30559 Hannover, Germany; [email protected] 3 Clinic for Cattle, Working Group Endocrinology, University of Veterinary Medicine Hannover, Bischofsholer Damm 15, 30173 Hannover, Germany; [email protected] 4 Dolfinarium, Zuiderzeeboulevard 22, 3841 WB Harderwijk, The Netherlands; paulien.bunskoek@dolfinarium.nl 5 Fjord & Bælt, Margrethes Pl. 1, 5300 Kerteminde, Denmark; [email protected] * Correspondence: [email protected]; Tel.: +49-511-856-8158 Simple Summary: The progress of animal welfare in wildlife conservation and research calls for more non-invasive sampling techniques. In cetaceans, exhaled breath condensate (blow)—a mixture of cells, mucus and fluids expelled through the force of a whale’s exhale—is a unique sampling matrix for hormones, bacteria and genetic material, among others. Especially the detection of steroid hormones, such as cortisol, is being investigated as stress indicators in several species. As the only Citation: Reckendorf, A.; Schmicke, native cetacean in Germany, harbor porpoises (Phocoena phocoena) are of special conservation concern M.; Bunskoek, P.; Anderson Hansen, and research interest. So far, strandings and live captures have been the only method to obtain K.; Thybo, M.; Strube, C.; Siebert, U. -

Full Text in Pdf Format
Vol. 6: 173–184, 2008 ENDANGERED SPECIES RESEARCH Printed December 2008 doi: 10.3354/esr00102 Endang Species Res Published online August 21, 2008 Contribution to the Theme Section ‘The IUCN Red List of Threatened Species: assessing its utility and value’ OPENPEN ACCESSCCESS Challenges of assessing cetacean population recovery and conservation status Milton M. R. Freeman* Canadian Circumpolar Institute, University of Alberta, Edmonton, Alberta T6G 2E1, Canada ABSTRACT: This paper examines the extent to which depleted whale populations have recovered (or not) and their perceived current conservation status and prognosis for continued survival, as repre- sented in the International Union for Conservation of Nature and Natural Resources (IUCN) Red Lists. It is concluded that current hypothetical and untested predictions of extinction risk, while in many cases drawing attention to justifiable conservation needs, may seriously undervalue the resilience of species that have evolved to live in a dynamic and ever-changing reality including cen- turies of heavy exploitation by humankind. The paper questions the appropriateness of Red Listing criteria for long-lived highly mobile ocean-dwelling species that are scarcely affected by the princi- pal threats upon terrestrial or aquatic species living in relatively restricted areas subject to habitat fragmentation and/or loss. This analysis draws attention to the problems associated with objectively assessing the conservation status of charismatic species and the value conflicts that may override evidence-based scientific conservation assessments. KEY WORDS: Whales · Whaling · Conservation · Population recovery · Predicting extinction · Red Listing · Charismatic species Resale or republication not permitted without written consent of the publisher INTRODUCTION of the bowhead whale Balaena mysticetus; both are believed to number in the tens or low hundreds at this The history of whaling provides one of the best- time. -

Ecological Factors Influencing Group Sizes of River Dolphins (Inia Geoffrensis and Sotalia Fluviatilis)
MARINE MAMMAL SCIENCE, **(*): ***–*** (*** 2011) C 2011 by the Society for Marine Mammalogy DOI: 10.1111/j.1748-7692.2011.00496.x Ecological factors influencing group sizes of river dolphins (Inia geoffrensis and Sotalia fluviatilis) CATALINA GOMEZ-SALAZAR Biology Department, Dalhousie University, Halifax, Nova Scotia B3H4J1, Canada and Foundation Omacha, Calle 86A No. 23–38, Bogota, Colombia E-mail: [email protected] FERNANDO TRUJILLO Foundation Omacha, Calle 86A No. 23–38, Bogota, Colombia HAL WHITEHEAD Biology Department, Dalhousie University, Halifax, Nova Scotia B3H4J1, Canada ABSTRACT Living in groups is usually driven by predation and competition for resources. River dolphins do not have natural predators but inhabit dynamic systems with predictable seasonal shifts. These ecological features may provide some insight into the forces driving group formation and help us to answer questions such as why river dolphins have some of the smallest group sizes of cetaceans, and why group sizes vary with time and place. We analyzed observations of group size for Inia and Sotalia over a 9 yr period. In the Amazon, largest group sizes occurred in main rivers and lakes, particularly during the low water season when resources are concentrated; smaller group sizes occurred in constricted waters (channels, tributaries, and confluences) that receive an influx of blackwaters that are poor in nutrients and sediments. In the Orinoco, the largest group sizes occurred during the transitional water season when the aquatic productivity increases. The largest group size of Inia occurred in the Orinoco location that contains the influx of two highly productive whitewater rivers. Flood pulses govern productivity and major biological factors of these river basins. -

THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A
s l a m m a y t T i M S N v I i A e G t A n i p E S r a A C a C E H n T M i THE CASE AGAINST Marine Mammals in Captivity The Humane Society of the United State s/ World Society for the Protection of Animals 2009 1 1 1 2 0 A M , n o t s o g B r o . 1 a 0 s 2 u - e a t i p s u S w , t e e r t S h t u o S 9 8 THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A. Rose, E.C.M. Parsons, and Richard Farinato, 4th edition Editors: Naomi A. Rose and Debra Firmani, 4th edition ©2009 The Humane Society of the United States and the World Society for the Protection of Animals. All rights reserved. ©2008 The HSUS. All rights reserved. Printed on recycled paper, acid free and elemental chlorine free, with soy-based ink. Cover: ©iStockphoto.com/Ying Ying Wong Overview n the debate over marine mammals in captivity, the of the natural environment. The truth is that marine mammals have evolved physically and behaviorally to survive these rigors. public display industry maintains that marine mammal For example, nearly every kind of marine mammal, from sea lion Iexhibits serve a valuable conservation function, people to dolphin, travels large distances daily in a search for food. In learn important information from seeing live animals, and captivity, natural feeding and foraging patterns are completely lost. -

Understanding Harbour Porpoise (Phocoena Phocoena) and Fisheries Interactions in the North-West Iberian Peninsula
20th ASCOBANS Advisory Committee Meeting AC20/Doc.6.1.b (S) Warsaw, Poland, 27-29 August 2013 Dist. 11 July 2013 Agenda Item 6.1 Project Funding through ASCOBANS Progress of Supported Projects Document 6.1.b Project Report: Understanding harbour porpoise (Phocoena phocoena) and fisheries interactions in the north-west Iberian Peninsula Action Requested Take note Submitted by Secretariat / University of Aberdeen NOTE: DELEGATES ARE KINDLY REMINDED TO BRING THEIR OWN COPIES OF DOCUMENTS TO THE MEETING Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Understanding harbour porpoise (Phocoena phocoena) and fishery interactions in the north-west Iberian Peninsula Fiona L. Read1,2, M. Begoña Santos2,3, Ángel F. González1, Alfredo López4, Marisa Ferreira5, José Vingada5,6 and Graham J. Pierce2,3,6 1) Instituto de Investigaciones Marinas (C.S.I.C), Eduardo Cabello 6, 36208 Vigo, Spain 2) School of Biological Sciences (Zoology), University of Aberdeen, Tillydrone Avenue, Aberdeen, AB24 2TZ, Aberdeen, United Kingdom 3) Instituto Español de Oceanografía, Centro Oceanográfico de Vigo, PO Box 1552, 36200, Vigo, Spain 4) CEMMA, Apdo. 15, 36380, Gondomar, Spain 5) CBMA/SPVS, Departamento de Biologia, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal 6) CESAM, Departamento de Biologia, Universidade do Aveiro, Campus Universitário de Santiago, 3810-193 Aveiro, Portugal Coordinated by: In collaboration with: 1 Final report to ASCOBANS (SSFA/ASCOBANS/2010/4) Introduction The North West Iberian Peninsula (NWIP), as defined for the present project, consists of Galicia (north-west Spain), and north-central Portugal as far south as Peniche (Figure 1). Due to seasonal upwelling (Fraga, 1981), the NWIP sustains high productivity and high biodiversity, including almost 300 species of fish (Solørzano et al., 1988) and over 75 species of cephalopods (Guerra, 1992). -

Order CETACEA Suborder MYSTICETI BALAENIDAE Eubalaena Glacialis (Müller, 1776) EUG En - Northern Right Whale; Fr - Baleine De Biscaye; Sp - Ballena Franca
click for previous page Cetacea 2041 Order CETACEA Suborder MYSTICETI BALAENIDAE Eubalaena glacialis (Müller, 1776) EUG En - Northern right whale; Fr - Baleine de Biscaye; Sp - Ballena franca. Adults common to 17 m, maximum to 18 m long.Body rotund with head to 1/3 of total length;no pleats in throat; dorsal fin absent. Mostly black or dark brown, may have white splotches on chin and belly.Commonly travel in groups of less than 12 in shallow water regions. IUCN Status: Endangered. BALAENOPTERIDAE Balaenoptera acutorostrata Lacepède, 1804 MIW En - Minke whale; Fr - Petit rorqual; Sp - Rorcual enano. Adult males maximum to slightly over 9 m long, females to 10.7 m.Head extremely pointed with prominent me- dian ridge. Body dark grey to black dorsally and white ventrally with streaks and lobes of intermediate shades along sides.Commonly travel singly or in groups of 2 or 3 in coastal and shore areas;may be found in groups of several hundred on feeding grounds. IUCN Status: Lower risk, near threatened. Balaenoptera borealis Lesson, 1828 SIW En - Sei whale; Fr - Rorqual de Rudolphi; Sp - Rorcual del norte. Adults to 18 m long. Typical rorqual body shape; dorsal fin tall and strongly curved, rises at a steep angle from back.Colour of body is mostly dark grey or blue-grey with a whitish area on belly and ventral pleats.Commonly travel in groups of 2 to 5 in open ocean waters. IUCN Status: Endangered. 2042 Marine Mammals Balaenoptera edeni Anderson, 1878 BRW En - Bryde’s whale; Fr - Rorqual de Bryde; Sp - Rorcual tropical. -

Download the PDF Article
palevolcomptes rendus 2020 19 5 DIRECTEURS DE LA PUBLICATION / PUBLICATION DIRECTORS : Bruno David, Président du Muséum national d’Histoire naturelle Étienne Ghys, Secrétaire perpétuel de l’Académie des sciences RÉDACTEURS EN CHEF / EDITORS-IN-CHIEF : Michel Laurin (CNRS), Philippe Taquet (Académie des sciences) ASSISTANTE DE RÉDACTION / ASSISTANT EDITOR : Adenise Lopes (Académie des sciences; [email protected]) MISE EN PAGE / PAGE LAYOUT : Martin Wable, Emmanuel Côtez (Muséum national d’Histoire naturelle) RÉDACTEURS ASSOCIÉS / ASSOCIATE EDITORS (*, took charge of the editorial process of the article/a pris en charge le suivi éditorial de l’article) : Micropaléontologie/Micropalaeontology Maria Rose Petrizzo (Università di Milano, Milano) Paléobotanique/Palaeobotany Cyrille Prestianni (Royal Belgian Institute of Natural Sciences, Brussels) Métazoaires/Metazoa Annalisa Ferretti (Università di Modena e Reggio Emilia, Modena) Paléoichthyologie/Palaeoichthyology Philippe Janvier (Muséum national d’Histoire naturelle, Académie des sciences, Paris) Amniotes du Mésozoïque/Mesozoic amniotes Hans-Dieter Sues (Smithsonian National Museum of Natural History, Washington) Tortues/Turtles Juliana Sterli (CONICET, Museo Paleontológico Egidio Feruglio, Trelew) Lépidosauromorphes/Lepidosauromorphs Hussam Zaher (Universidade de São Paulo) Oiseaux/Birds Éric Buffetaut (CNRS, École Normale Supérieure, Paris) Paléomammalogie (petits mammifères)/Palaeomammalogy (small mammals) Robert Asher (Cambridge University, Cambridge) Paléomammalogie (mammifères -
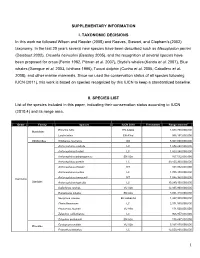
Global Patterns in Marine Mammal Distributions
SUPPLEMENTARY INFORMATION I. TAXONOMIC DECISIONS In this work we followed Wilson and Reeder (2005) and Reeves, Stewart, and Clapham’s (2002) taxonomy. In the last 20 years several new species have been described such as Mesoplodon perrini (Dalebout 2002), Orcaella heinsohni (Beasley 2005), and the recognition of several species have been proposed for orcas (Perrin 1982, Pitman et al. 2007), Bryde's whales (Kanda et al. 2007), Blue whales (Garrigue et al. 2003, Ichihara 1996), Tucuxi dolphin (Cunha et al. 2005, Caballero et al. 2008), and other marine mammals. Since we used the conservation status of all species following IUCN (2011), this work is based on species recognized by this IUCN to keep a standardized baseline. II. SPECIES LIST List of the species included in this paper, indicating their conservation status according to IUCN (2010.4) and its range area. Order Family Species IUCN 2010 Freshwater Range area km2 Enhydra lutris EN A2abe 1,084,750,000,000 Mustelidae Lontra felina EN A3cd 996,197,000,000 Odobenidae Odobenus rosmarus DD 5,367,060,000,000 Arctocephalus australis LC 1,674,290,000,000 Arctocephalus forsteri LC 1,823,240,000,000 Arctocephalus galapagoensis EN A2a 167,512,000,000 Arctocephalus gazella LC 39,155,300,000,000 Arctocephalus philippii NT 163,932,000,000 Arctocephalus pusillus LC 1,705,430,000,000 Arctocephalus townsendi NT 1,045,950,000,000 Carnivora Otariidae Arctocephalus tropicalis LC 39,249,100,000,000 Callorhinus ursinus VU A2b 12,935,900,000,000 Eumetopias jubatus EN A2a 3,051,310,000,000 Neophoca cinerea -

Vaquitas and Gillnets: Mexico’S Ultimate Cetacean Conservation Challenge
Vol. 21: 77–87, 2013 ENDANGERED SPECIES RESEARCH Published online July 3 doi: 10.3354/esr00501 Endang Species Res Contribution to the Theme Section: ‘Techniques for reducing bycatch of marine mammals in gillnets’ FREEREE ACCESSCCESS Vaquitas and gillnets: Mexico’s ultimate cetacean conservation challenge Lorenzo Rojas-Bracho1,*, Randall R. Reeves2 1Coordinación de Investigación y Conservación de Mamíferos Marinos, Instituto Nacional de Ecología y Cambio Climático, C/o CICESE Carretera Ensenada-Tijuana 3918, Ensenada BC 22860, México 2Okapi Wildlife Associates, 27 Chandler Lane, Hudson, Quebec J0P 1H0, Canada ABSTRACT: There is a high risk that incidental mortality (bycatch) in gillnets will lead to extinc- tion of the vaquita Phocoena sinus, a small porpoise endemic to Mexico’s northern Gulf of Califor- nia. A zoned Biosphere Reserve established in 1993 proved ineffective at slowing the population’s decline, and in 2005, a Vaquita Refuge was declared. The Refuge Program included a ban on gill- netting and trawling in certain areas with relatively high densities of vaquitas. However, it was not until 2008, with the introduction of a Species Conservation Action Plan for Vaquita (PACE- Vaquita), that a comprehensive protection and recovery effort was introduced. Unfortunately, valuable time was lost as officials first needed to bring order to a poorly managed fishery manage- ment system. Also, the voluntary nature of fisherman participation and the chronic deficiency of enforcement have limited PACE’s effectiveness. Although efforts to implement the plan probably slowed the vaquita’s decline, the goal of eliminating gillnets (and thus most vaquita bycatch) by 2012 was not reached. This example shows the difficulty of achieving conservation when the pro- gram’s rationale centers on the preservation of biodiversity with less emphasis on meeting com- munity aspirations.