Boctor of $I)Uo£;Opt)P in GEOLOGY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Population Statistics, 4-Meerut, Uttar Pradesh
I Census of India, 195 1 DISTRICT POPULATION STATISTICS UTTAR PRADESH 4-MEEl{UT DISTRICT 315.42 ALLAHABAD: TING AND STATIONERY, UTTAR PRADESH, INDIA 1951 1952 MEE DPS Price, Re.1-S. FOREWORD THE Uttar Pradesh Government asked me in March. 1952, (0 'supply them for the purposes of elections to local bodies population statistics with ,separation for scheduled castes (i) mohalla/ward-wise for urban areas, and (ii) village-wise for rural areas. The Census Tabulation Plan did nbt provide for sorting of scheduled cast<;s population for areas smaller than a tehsil or urban tract and the request from the Uttar Pradesh Government came when the slip sorting had been finished and (he Tabulation Offices closed. As the census slips are mixed up for the purposes of sorting in one lot for a tehsil or urban tract, collection of data regarding scheduled castes population by moh'allas/wards and villages would have involved enormous labour and expense if sorting of the slips had been taken up afresh. Fortunately, however, a secondary census record, viz. the National Citizens' Register, in which each slip has been copied, was available. By singular foresight it had been pre pared mohalla/ward-wise for urban areas and village-wise for rural areas. Th e required information has, therefore. been extracted from. this record, 2. In the above circumstances there is a slight difference in the figures of population as arrived at by an earlier sorting of the slips and as now determined by counting from the National Citizens' Register. This difference has been accen mated by an order passed by me during the later coum from the National Register of Citizens as follows:- (i) Count Ahirwars of Farrukhabad District, Raidas and Bhagar as ·Chamars'. -

Bagpat Page:- 1 Cent-Code & Name Exam Sch-Status School Code & Name #School-Allot Sex Part Group 1001 Janta Inter College Palari Bagpat Brm
DATE:27-02-2021 BHS&IE, UP EXAM YEAR-2021 **** FINAL CENTRE ALLOTMENT REPORT **** DIST-CD & NAME :- 13 BAGPAT PAGE:- 1 CENT-CODE & NAME EXAM SCH-STATUS SCHOOL CODE & NAME #SCHOOL-ALLOT SEX PART GROUP 1001 JANTA INTER COLLEGE PALARI BAGPAT BRM HIGH BRM 1001 JANTA INTER COLLEGE PALARI BAGPAT 61 F HIGH BRM 1005 J K INTER COLLEGE DHANOURA TIKRI BAGPAT 45 M HIGH BRM 1010 J S INTER COLLEGE NIRPUDA BAGPAT 41 M HIGH BRM 1012 HARCHANDMAL JAIN INT COLL TIKRI BAGPAT 140 M HIGH CRM 1015 A V INT COLL JEBABAD KHAPRANA BAGPAT 102 F HIGH CRM 1135 O B S HR SEC SCHOOL BARNAWA BAGPAT 164 M - 553 INTER BRM 1001 JANTA INTER COLLEGE PALARI BAGPAT 71 F ALL GROUP INTER BRM 1005 J K INTER COLLEGE DHANOURA TIKRI BAGPAT 3 M OTHER THAN SCICNCE INTER BRM 1005 J K INTER COLLEGE DHANOURA TIKRI BAGPAT 11 F OTHER THAN SCICNCE INTER BRM 1009 SHRI JAWAHAR INT COLL BAMNOLI BAGPAT 30 M OTHER THAN SCICNCE INTER BRM 1012 HARCHANDMAL JAIN INT COLL TIKRI BAGPAT 63 M OTHER THAN SCICNCE INTER BRM 1012 HARCHANDMAL JAIN INT COLL TIKRI BAGPAT 163 M SCIENCE INTER CRM 1015 A V INT COLL JEBABAD KHAPRANA BAGPAT 26 F OTHER THAN SCICNCE INTER CRM 1015 A V INT COLL JEBABAD KHAPRANA BAGPAT 71 F SCIENCE INTER CRM 1126 N S C BOSS MEMO I C TAVELAGARHI BAGPAT 34 F ALL GROUP INTER CRM 1135 O B S HR SEC SCHOOL BARNAWA BAGPAT 14 F OTHER THAN SCICNCE INTER ARF 5003 GOVT GIRLS INTER COLLEGE DAHA BAGPAT 59 M ALL GROUP 545 CENTRE TOTAL >>>>>> 1098 1002 ARYA VIDYALAYA INTER COLLEGE TERA BAGPAT BRM HIGH BRM 1002 ARYA VIDYALAYA INTER COLLEGE TERA BAGPAT 29 F HIGH BRM 1013 S A V INTER COLLEGE KAMALA JUR -

Uttar Pradesh
Uttar Pradesh RPS ITC UPZJ7C Name Ram Piyare Singh Industrial Training Centre Address Maharwa Gola , , , Ambedkar Nagar - File Nos. DGET-6/24/16/2003-TC Govt. ITI UPZJ8C Name Govt. Industrial Training Institute, Tanda Address Tanda , , , Ambedkar Nagar - File Nos. DGET-6/24/7/2001-TC Chandra Audyogik UPZLPX Name Chandra Audyogik Prashikshan Kendra Address Dhaurhara, Sinjhauli , , , Ambedkar Nagar - File Nos. DGET-6/24/152/2009-TC WITS ITC UPZLQ4 Name WITS ITC Address Patel Nagar Akbarpur , , , Ambedkar Nagar - 224122 File Nos. DGET-6/24/160/2009-TC Kamla Devi Memorial Voc. UPZLT9 Name Kamla Devi Memorial Vocational Training Institute Address Pura Baksaray, Barua Jalaki, Tanda , , , Ambedkar Nagar - File Nos. DGET-6/24/235/2009-TC Hazi Abdullah ITC UPZLTK Name Hazi Abdullah ITC Address Sultanpur Kabirpur, Baskhari , , , Ambedkar Nagar - File Nos. DGET-6/24/238/2009-TC K.B.R ITC UPZM02 Name K.B.R ITC Address Shastri Nagar, Akbarpur , , , Ambedkar Nagar - File Nos. DGET-6/24/236/2009-TC Govt.ITI (W) Agra UP1750 Name Govt. Industrial Training Institute (Women Branch) Address , , , Agra - 0 File Nos. 0 Women Govt ITI, Agra UP1751 Name Govt. Industrial Training Institute for Women (WB) Address Vishwa Bank , , , Agra - 0 File Nos. DGET-6/24/16/2000-TC Govt ITI Agra UP1754 Name Govt Industrial Training Institute Address , , , Agra - 282001 File Nos. DGET-6/24/20/92 - TC Fine Arts Photography Tra UP2394 Name Fine Arts Photography Training Institute Address Baba Bldg. Ashok Nagar , , , Agra - 282001 File Nos. DGET-6/21/1/88 - TC National Instt of Tech Ed UPZJZ2 Name National Institute of Tech Educational Vijay Nagar Colony Address North Vijay Nagar Colony , , , Agra - 282004 File Nos. -
District Census Handbook, Meerut, Part X-A, Series-21, Uttar Pradesh
CENSUS 1971 PART X-A Tcr\VN< & VILLAGE DIRECTORY SERIES 21 UTTAR PRADESH DISTRICT. DISTRICT MEERUT CENSUS HANDBOOK D. M. SINHA OF THE i};DIAN AD1IlNISTRATIVE SERVICE Director of Census Operatiorn Uttar Pradesh DISTRICT MEERUT I 10 I) 10 KMS b:.u.=.:.- -± - - 1--±=:;d o ". IL- f- i ,<-lS 01STRICT 1l0UNOARY TAHSIL BOUNDARY 'YIKAS ~HflND IIOUNDARY DISTRICT HEAOQUARTERS TAHSIL HEA.OQUA.RnR~ I""" ~ VtKIS KHA.Ha H~AOQU"'fHkS .~".'"' ,." 10111101 OF THE DIITRICT o ,v • ,.~\ ',., IN UTTAR PRIOEIH URBAN IUfA f/ c'~"'\f/ IJ . ~ - \, ,. "\ VILI.AGE WITH POPULATION MI]lI Olt "1011£ • ~~,' :'\ 0 IO::J 200 .(\,~S HIGHWAYS. NA1'IONAL, ,TATE l~iltUL_ )..'1:) r'; ~ OTHER IMPORTANT ROAD' ' ____ .- I R.A1L'hAV UI\IE WITH STAttON. BROAD (iIl.UC.EI, __ "i~ .... _ Nome of the A,,, in IPoPUIO\iOn No." No. of NARROW"A.UGEI~_ ,\. Tahsil K.' Villagfs Towns v;:-.... RIVER AND 5TRfAH I " ........ '" ~),. BlGHPII 1,0lll 561,066 154 CANAL WI1l11MPORTANT DISTRIBUiflfW \ I GHIZIIBAD 1.0581 718.91J III POlICf STATION P5 IIROHINI 895·1 4\M11 106 ron & nLEG.RA~H OFFICe. I PI MEERUI 7110 141.B14 119 RtH HOUSi TRAVELLERS' BUNGALOW, HC, I RH 5" HAmA 1.098.4 J90.))5 l06 HOSPITAL, PlSPENSARY,P, H, CENnE, ETC + HAPUR 1.0811 516.73B ll, DEGREE (OLlEG£, H. S, SCHOOL 8,0 TOTAL 5,944.0 3,%6.951 1,651 22 L_·--~~~~~-o~,--------~------------~~------~----~----~---, , 77 15 East of Gr"cw", 30 ~5 CONTENTS Pages Acknowledgements Introductory Note iii TOWN AND VILLA.GE DIRECTORY Town Directory Statement I-Status, Growth History and Functional Category of Towns 4-5 Statement II-Physical Aspects -

Outcome Budget 2015
GOVERNMENT OF INDIA OUTCOME BUDGET 2015 - 2016 MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT & GANGA REJUVENATION CONTENTS Chapter/Para Aspect Page(s) No. EXECUTIVE SUMMARY I BRIEF INTRODUCTORY NOTE ON THE 1-14 FUNCTIONS OF THE MINISTRY / DEPARTMENT, ORGANIZATIONAL SET UP, LIST OF MAJOR PROGRAMMES / SCHEMES IMPLEMENTED BY THE MINISTRY / DEPARTMENT, ITS MANDATE, GOALS AND POLICY FRAMEWORK II STATEMENT OF OUTLAYS AND 15-29 OUTCOMES/ TARGETS: ANNUAL PLAN 2015-16 III REFORM MEASURES AND POLICY 30-38 INITIATIVES IV REVIEW OF PAST PERFORMANCE 39 V OVERALL FINANCIAL REVIEW 40-50 Trend of expenditure in FY 2014-15 40-41 Budget at a Glance 42-49 Utilization Certificates 50 VI REVIEW OF PERFORMANCE OF STATUTORY /AUTONOMOUS ORGANISATIONS AND PUBLIC SECTOR UNDERTAKINGS Statutory Bodies: 6.1.1-6.1.2 Brahmaputra Board 51-54 6.2 Ravi and Beas Waters Tribunal 54 6.3 Cauvery Water Disputes Tribunal 55-56 6.4 Krishna Water Disputes Tribunal 56-57 6.5 Vansadhara Water Disputes Tribunal 57-58 6.6 Mahadayi Water Disputes Tribunal 58-60 6.7 Godavari and Krishna River Management Boards 60-61 Autonomous Bodies (Societies): 6.8 National Water Development Agency 61-63 i 6.9 National Institute of Hydrology 63-65 Public Sector Undertakings: 6.10 Water and Power Consultancy Services (India) 65-67 Limited 6.11 National Projects Construction Corporation Limited 67-69 ANNEXURE I Performance of 2013-14 70-95 II Performance of 2014-15 96-138 III Information regarding Accelerated Irrigation 139-146 Benefits Programme & National Project IV Statement showing details of Ministry of Water 147 Resources budget vis-a-vis XI Plan Outlay V Statement showing details of Ministry of Resources 148-149 Budget vis-à-vis XII Plan Outlay. -
ITI Code ITI Name ITI Category Address State District Phone Number Email Name of FLC Name of Bank Name of FLC Mobile No
ITI Code ITI Name ITI Category Address State District Phone Number Email Name of FLC Name of Bank Name of FLC Mobile No. Of Landline of Address Manager FLC Manager FLC GR09000145 Karpoori Thakur P VILL POST GANDHI Uttar Ballia 9651744234 karpoorithakur1691 Ballia Central Bank N N Kunwar 9415450332 05498- Haldi Kothi,Ballia Dhanushdhari NAGAR TELMA Pradesh @gmail.com of India 225647 Private ITC - JAMALUDDINPUR DISTT Ballia B GR09000192 Sar Sayed School P OHDARIPUR, Uttar Azamgarh 9026699883 govindazm@gmail. Azamgarh Union Bank of Shri R A Singh 9415835509 5462246390 TAMSA F.L.C.C. of Technology RAJAPURSIKRAUR, Pradesh com India Azamgarh, Collectorate, Private ITC - BEENAPARA, Azamgarh, 276001 Binapara - AZAMGARH Azamgarh GR09000314 Sant Kabir Private P Sant Kabir ITI, Salarpur, Uttar Varanasi 7376470615 [email protected] Varanasi Union Bank of Shri Nirmal 9415359661 5422370377 House No: 241G, ITC - Varanasi Rasulgarh,Varanasi Pradesh m India Kumar Ledhupur, Sarnath, Varanasi GR09000426 A.H. Private ITC - P A H ITI SIDHARI Uttar Azamgarh 9919554681 abdulhameeditc@g Azamgarh Union Bank of Shri R A Singh 9415835509 5462246390 TAMSA F.L.C.C. Azamgarh AZAMGARH Pradesh mail.com India Azamgarh, Collectorate, Azamgarh, 276001 GR09001146 Ramnath Munshi P SADAT GHAZIPUR Uttar Ghazipur 9415838111 rmiti2014@rediffm Ghazipur Union Bank of Shri B N R 9415889739 5482226630 UNION BANK OF INDIA Private Itc - Pradesh ail.com India Gupta FLC CENTER Ghazipur DADRIGHAT GHAZIPUR GR09001184 The IETE Private P 248, Uttar Varanasi 9454234449 ietevaranasi@rediff Varanasi Union Bank of Shri Nirmal 9415359661 5422370377 House No: 241G, ITI - Varanasi Maheshpur,Industrial Pradesh mail.com India Kumar Ledhupur, Sarnath, Area Post : Industrial Varanasi GR09001243 Dr. -

Notification for the Posts of Gramin Dak Sevaks Cycle – Iii/2021-2022 Uttar Pradesh Circle
NOTIFICATION FOR THE POSTS OF GRAMIN DAK SEVAKS CYCLE – III/2021-2022 UTTAR PRADESH CIRCLE RECTT/GDS ONLINE ENGAGEMENT/CYCLE-III/UP/2021/8 Applications are invited by the respective engaging authorities as shown in the annexure ‘I’against each post, from eligible candidates for the selection and engagement to the following posts of Gramin Dak Sevaks. I. Job Profile:- (i) BRANCH POSTMASTER (BPM) The Job Profile of Branch Post Master will include managing affairs of Branch Post Office, India Posts Payments Bank ( IPPB) and ensuring uninterrupted counter operation during the prescribed working hours using the handheld device/Smartphone/laptop supplied by the Department. The overall management of postal facilities, maintenance of records, upkeep of handheld device/laptop/equipment ensuring online transactions, and marketing of Postal, India Post Payments Bank services and procurement of business in the villages or Gram Panchayats within the jurisdiction of the Branch Post Office should rest on the shoulders of Branch Postmasters. However, the work performed for IPPB will not be included in calculation of TRCA, since the same is being done on incentive basis.Branch Postmaster will be the team leader of the Branch Post Office and overall responsibility of smooth and timely functioning of Post Office including mail conveyance and mail delivery. He/she might be assisted by Assistant Branch Post Master of the same Branch Post Office. BPM will be required to do combined duties of ABPMs as and when ordered. He will also be required to do marketing, organizing melas, business procurement and any other work assigned by IPO/ASPO/SPOs/SSPOs/SRM/SSRM and other Supervising authorities. -
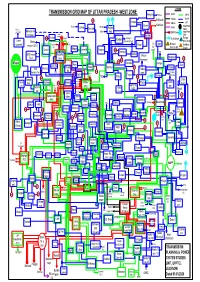
C:\Users\DELL\Desktop\Prashant
LEGEND TRANSMISSION GRID MAP OF UTTAR PRADESH -WEST ZONE Manglore Roorkee HVDC 220 kV 38 765 kV 132 kV 54 5 Rishikesh Kiratpur 400 kV U/C Morna Kashipur Solar Plant Roorkee Laksar Kotdwar Najiba 66 kV Roorkee Chandok 132kV 220/132kV Mawana Rd. Chilla bad 132kV C0-Gen Solar Plant Vishnu 33kV Hastinapur prayag. Nehtaur 48 Purkazi Alaknanda Kalagarh 33 kV C0-Gen Flue gas Hydro Afzalgarh Laxmi Bhag Ramganga G.S. base Co-gen To C.B Lalpur Biomass sugarmill wanpur (U.K.) Roorkee Srinagar Bijnor Coal Base PG Ganj base Co-gen Co-gen Charla Bhopa Janseth Nagina 29 220kV Dhampur TSS SRE Ambala Muzaffar Sherkot Mahua Rd.-II nagar Roorkee Shahabad 51 67 Kheraganj 21 Chandpur Rampur Khara Pura Bilari Jolly Rd. Bhopa Nara Nehtaur 27 Bilaspur SRE Ramraj Thakurd TSS Rd 31 C.B Ganj Ambala Rd.-I Bareilly wara 10 Baghra (PG) Tajpur TSS 220/132kV TSS Jalilpur 56 58 Rampur Gangal 14 Moga Chan Chutmal Koteshwar Mataur Gulab heri Sarsawa 20 Khatauli Mandola Tanda duasi Roorkee (PG) Kanth Rd. pur Saharan (PG) bari PG Avas MBD Bisauli pur Khodri Lalu Bhivani Vikas 62 59 Saharanpur Kheri Salava Kankar Kundarkhi khera-II (PG) (Kapsad) Modi Agwanpur TSS Gajraula Kashipur Moradabad Babrala Nakur Kota puram Sardhana Jhinjana Mawana 40 India 49 TSS 8 Garhmuk Designco Glycols 24 Amroha Behjoi Rampur 13 Ganganagar Gajraula teshwar Amroha 1MW Deoband Kankar Bachraun Bareilly Maniharan 41 42 khera 37 (PG) Sahas Behat Thanabhawan 33 wan Chan Gangoh Medical Col. Kothi (Kalsia) (Jalalabad) 17 duasi Budhana Vedvyas khidmatpur Nagli 3 Badaun puri kithore Simbholi Sambhal Nanuta 63 Sambhal Bannat Shamli Shatabdi B B Nagar 72 To Shamli Shyamla Nagar Saidnagli Dehradun Mundali Jagritivihar TSS Hapur Siyana Kharad Asmauli 57 Nirpura Kaniyan Hapur Rd. -

II/2019 UTTAR PRADESH CIRCLE Applications Are Invited by the Respe
NOTIFICATION FOR THE POSTS OF GRAMIN DAK SEVAKS CYCLE – II/2019 UTTAR PRADESH CIRCLE RECTT/GDSRECTT/GDS ONLINE ONLINE ENGAGEMENT/UP/2020/8 ENGAGEMENT/UP/2020/8 DATED 23.03.2020 Applications are invited by the respective recruiting authorities as shown in the annexure ‘I’against each post, from eligible candidates for the selection and engagement to the following posts of Gramin Dak Sevaks. I. Job Profile:- (i) BRANCH POSTMASTER (BPM) The Job Profile of Branch Post Master will include managing affairs of GDS Branch Post Office, India Posts Payments Bank (IPPB) and ensuring uninterrupted counter operation during the prescribed working hours using the handheld device/Smartphone supplied by the Department. The overall management of postal facilities, maintenance of records, upkeep of handheld device, ensuring online transactions, and marketing of Postal, India Post Payments Bank services and procurement of business in the villages or Gram Panchayats within the jurisdiction of the Branch Post Office should rest on the shoulders of Branch Postmasters. However,the work performed for IPPB will not be included in calculation of TRCA, since the same is being done on incentive basis.Branch Postmaster will be the team leader of the GDS Post Office and overall responsibility of smooth and timely functioning of Post Office including mail conveyance and mail delivery. He/she might be assisted by Assistant Branch Post Master of the same GDS Post Office. BPM will be required to do combined duties of ABPMs as and when ordered. He will also be required to do marketing, organizing melas, business procurement and any other work assigned by IPO/ASPO/SPOs/SSPOs/SRM/SSRM etc.In some of the Branch Post Offices, the BPM has to do all the work of BPM/ABPM. -

MEERUT: 111111111111'1111111111111111111111111111 Gfpe-PUNE-017942 a GAZETTEER
Dhauanjayarao Gadgii LibRaRy MEERUT: 111111111111'1111111111111111111111111111 GfPE-PUNE-017942 A GAZETTEER, llEING VOLUME IV OF THE DISTRICT GAZETTEERS OF THE UNITEll PROVINCES OF AGRA AND OUDH. COMPILED A.ND EDITED :BY H. R. N E V ILL, 1. C. S. ALLAHABAD: PRINTED :BY F. LUKER, SUl/DT., GOVT. PRESS, UNiTED PnOTINCES. 1904. Price Rs. 3 (48.). GAZETTEER OF MEERU~i .CONTENTS.,. PAGE, .,~AGE, Occupp.tions ••• 101 Boundaries and Area 1 Villages and houses , . 102 Topography ... 2 Contlition of the people 105 },akes · . .. 18 Proprietor~· ••• . ., 107 Tenants; ... , 108 Waste lands •· · ... ', 1' ... 19 ... Groves 21 Rents . ... 109 Minerals ~··... 22 Language and Literature •• , 109 }'auna 24 Edu<(ation : .. , ... 110 Cattle 26 ~· Climate and Rainfall 29 CRAPTEB IV. Medical Aspects ... ... 31 District Sta:ff ... J.I5 CH!PT~]I1I• . Garrison ... 116' Subdivisions 116 Cultivation .. •.. 35 .... Fiscal History ' ... 119 ~oils .. .... 37'' Police' 136 Harvests · ;.. ••.•. 38 Crime 138 Crops .: •. •··· 3~ ,Jail' , '. ·140 .., .. 47 .. l~ri~atiol\ and Cana~s. ~- '·"' 140 .... 57• :Excise ..•. ... }amines ., ·•'• .~ Registratj.on .... ~· .... 142 J>rices andWag~8' .~ •. ·::..~·· 60 .Stamps .. ... ·Ha:. Trade .. • , .. ..... 61 lncome-tax . ' 143 }'airs . •.. .. .... 63 Post-offi!leS . '143 Weights and Mej;\sures 63 Municipalities ... ']44. ]nterest · ·: ~ :' :.,: l.. 64 Act XX towns ... ' ... 145 Manufactures ;,,.' .• · · ":· 65 District Board· ... ... 145 <:ommunicati~n{ .".:... ·:.· :·: ;1 ... 67 _l)i s pensarie s ••• 141i . ... C:S:Al'TEB ·ll[ ' ' ·. CriPTER V. ; '75 Population •. ·.. ~-.~~ r~'. '·•·•"' ~l'l. • ··41 . '147 .. , . 78. Uis~ory ··~... ...... '• , ... 78 -~ J~,·li:;-ions- · ' ... ,, 79 ~ 187 (.'bri~~ianity __:' . ~. ~ . Directory ... ... ,....._...:... '"· Arya Sam~j . · .... ' ,,, 82;. <'' .hins .... .... .,, 82 Appen<U~ ... ·t. .. ·i-ilviii .. I • ,...___ ?\I u sal uia D.~ : ... ..... 83 lliuJue ... ... 88 Index~ ••• ,.... i...;.vii PREFACE. -

District Census Handbook, 4-Meerut, Uttar Pradesh
, ; : \ ,r , ! '\ ' ,.. c· tl'l'rlWDtTOTJON- A-The Di.triot i-III B-AnaJysia of the Stati.tiOB iii-Ii C-Explanatory notes on the Statistics xi-xiii PART I-DISTRICT CENSUS TABLES A-'.}'I!:N'ER.A.L POPULATION TABLES-- _ A-I _ Are.. , Houses Bnd Population 3 A·II Variation in Population during Fifty y"...... 3 A-III Towns and Villages Classified by Population 4-5 A-Tv Towns CI"""ifled by Population with Variations since 1901 6-8 JV Towns ..'ranged Territorially with Population by Livelihood C1asse. 9 E AT.". and Populotion of Di.triot and TollSil. by Livelihood ChwseB 10-11 B-EooNollUo TA1ILES- B-! Llvalihood OIasses and Sub,olasses 12-17 B-II Sooondary ~ean" of LiveHhood .': 18-33 B-III Employers, Employees and Indepe';dent Workers in Industrie~ and Services by Divisions and Snb-divisions '_ 34-62 B-IV Unemployment by Livelihood Classes 63-64 .~". Index or Non,agrioultural Oooupations 65-69 \, O-HOtTSEltOLD AND AGE (SA>C'LB) TA1ILBS- 0-1 Household (Size and Composition) 70-71 O-II Livelihood Olasses by Age-groups 72-79 O-III Age";"d Civil Condition 80-83 C-IV Age and Liter...,y 84-87 C-V Single Year Age Returns __ 88-95 l>-Soor.u. AND CUW!UBAL T"'-BLES- - - } Languages <il Mother Tongue 96-91· • <iiI Bilingualism 98-101 n-H Religion r_ 102-103 D-HI Soheduled Castes 102-103, n-IV Migrants •• ' 104-107 nov (i) Displaoed persons by year of arriva11n India 108-109 (ii) Displa.ced pe':"'ns by Livelihood Cl_ tiO-111 n-VI Non-Indian NatIonals _O., 1I0-1! I n-VII Livelihood Olasseo hy EduoationalSt.andardo 112-117 PART II-VILLAGE, TOWN, PARGANA AND THANA STATISTICS I Primary Census Abstraot 120-191 2 P"rgana and Than&-wi1!6 Population 192-193 PART m-MISCELLANEOUS STATISTICS I Vltol Statlatioa 196-199 2 Agricultural Ststlatlos-(i) Rainfall •• '_:J 200-201 (iiI Area 88 ol ....ifled with detaila of area under cUltivation 202-205 (iii) Cropped Area 206-221 (iv) Irrigated A .... -

MRT Brick Kiln Status on 28814
List Of Brick Kiln According to Meerut Regional Office U.P Pollution Control Board Record Status As on 28.8.2014 {ks=h; dz0la0 tuin bZaV HkV~Bks dk uke irs lfgr ,uvkslh@ ,uvkslh@ ,uvkslh@ lgefr uksfVl@canh vkns'k fuxZr djus ifjokn dk;Zky; lgefr lgefr tkjh lgefr dc ls dk;Zokgh@frfFkdh fLFkfr frfFk lfgr laca/kh dk uke vkosnu djus dh vkosnu dc rd fooj.k ;fn i= izzkfIRk frfFk vLohd`r ifjokn dh frfFk djus laca/kh nk;j fd;k Meerut 1 Meerut Anawari Brick Field, Mahmoodpur Sikhera, Bahsuma, 10.3.2010 19/05/2010 2014 Meerut Meerut 2 Meerut Arun Brick Works, Hasanpur Kadeem, Garh Road, Meerut 18.3.2010 30/06/2010 2014 Meerut 3 Meerut Atul Brick Works, Murlipur, Garh Road, Meerut 18.3.2010 30/06/2010 2014 Meerut 4 Meerut Adarsh Brick Filed, Ataura, Mawana, Meerut 20.3.2010 19/05/2010 2014 Meerut 5 Meerut Anand Brick Field, Sikheda, mawana, Meerut 20.3.2010 19/05/2010 2014 Meerut 6 Meerut Apna Brick Field, Puthi, Mawana, Meerut 20.3.2010 19/05/2010 2014 Meerut 7 Meerut Arora Brick Field, Ukhlina, Kalyanpur, Meerut 26.3.2010 19/05/2010 2014 Meerut 8 Meerut ABF Associates, Bahrampur, Khas, Meerut 26.3.2010 19/05/2010 2014 Meerut 9 Meerut Ashoka Brick Field, Sindhawali, Rohta road, Meerut 29.3.2010 23/07/2010 2014 Meerut 10 Meerut Ambey Brick Field, Kudi Hapur Road, Meerut 31.3.2010 30/06/2010 2014 Meerut 11 Meerut Amit Brick Field, Sisauli, Garh Road, Meerut 28.3.2010 07/06/2010 2014 Meerut 12 Meerut Ambey Brick Field, Jeetpur, Sakauti, Meerut 31.3.2010 19/05/2010 2014 Meerut 13 Meerut Amar Bhatta Co.