Equations of State and Spinodal Lines a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Van Der Waals Force & Hydrophobic Effect
International Journal of Advanced Computer Technology (IJACT) ISSN:2319-7900 Van der Waals Force & Hydrophobic Effect Park, Ho-Min, Grade 12, Ewell Castle School , UK. [Abstract] polar atoms interacting with each other, Deybe Force Van der Waals forces are electrostatic forces. They Effect which caused between a molecule with is polar, operate not only between polar molecules but also and one that is not, and London Dispersal Effect that acts between electrically neutral atoms and molecules. This is between two non-polar molecules/atoms[3]. Because the because the movement of electrons in the outer shell of electrons around each molecule/atom repel each other, it the atoms temporarily leads to charge displacements – creates a redistribution of charge, inducing an and to so-called polarization. Charged areas with instantaneous dipole moment[3]. Dispersion forces are different signs are then attracted to one another – and caused by fluctuations in the electron distribution within therefore ensure an attraction between two atoms, even if molecules or atoms. Since all atoms and molecules have these are electrically neutral overall. The van der Waals electrons, they all have dispersion forces. The electrons force is an electromagnetic interaction between in an atom or molecule may, at any one instant, be correlated fluctuating charges on two electrically neutral unevenly distributed[4]. The purpose of this study is to surfaces. As the surfaces approach more closely, the explore mechanisms of van der Waals forces forces and force increases as fluctuations of shorter and shorter to identify the potential on the scientific field. length scale come into play, but ultimately the force will saturate when the surfaces are so close that the even shortest wavelength charge fluctuations are included. -

Phase Diagrams and Phase Separation
Phase Diagrams and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon P Haasen, Physical Metallurgy, G Strobl, The Physics of Polymers, Springer Introduction Mixing two (or more) components together can lead to new properties: Metal alloys e.g. steel, bronze, brass…. Polymers e.g. rubber toughened systems. Can either get complete mixing on the atomic/molecular level, or phase separation. Phase Diagrams allow us to map out what happens under different conditions (specifically of concentration and temperature). Free Energy of Mixing Entropy of Mixing nA atoms of A nB atoms of B AM Donald 1 Phase Diagrams Total atoms N = nA + nB Then Smix = k ln W N! = k ln nA!nb! This can be rewritten in terms of concentrations of the two types of atoms: nA/N = cA nB/N = cB and using Stirling's approximation Smix = -Nk (cAln cA + cBln cB) / kN mix S AB0.5 This is a parabolic curve. There is always a positive entropy gain on mixing (note the logarithms are negative) – so that entropic considerations alone will lead to a homogeneous mixture. The infinite slope at cA=0 and 1 means that it is very hard to remove final few impurities from a mixture. AM Donald 2 Phase Diagrams This is the situation if no molecular interactions to lead to enthalpic contribution to the free energy (this corresponds to the athermal or ideal mixing case). Enthalpic Contribution Assume a coordination number Z. Within a mean field approximation there are 2 nAA bonds of A-A type = 1/2 NcAZcA = 1/2 NZcA nBB bonds of B-B type = 1/2 NcBZcB = 1/2 NZ(1- 2 cA) and nAB bonds of A-B type = NZcA(1-cA) where the factor 1/2 comes in to avoid double counting and cB = (1-cA). -

Suppression of Phase Separation in Lifepo4 Nanoparticles
Suppression of Phase Separation in LiFePO4 Nanoparticles During Battery Discharge Peng Bai,†,§ Daniel A. Cogswell† and Martin Z. Bazant*,†,‡ †Department of Chemical Engineering and ‡Department of Mathematics, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, Massachusetts 02139, USA §State Key Laboratory of Automotive Safety and Energy, Department of Automotive Engineering, Tsinghua University, Beijing 100084, P. R. China *Corresponding author: Dr. Martin Z. Bazant. Tel: (617)258-7039; Fax: (617)258-5766; Email: [email protected] August 8, 2011 ABSTRACT: Using a novel electrochemical phase-field model, we question the common belief that LixFePO4 nanoparticles separate into Li-rich and Li-poor phases during battery discharge. For small currents, spinodal decomposition or nucleation leads to moving phase boundaries. Above a critical current density (in the Tafel regime), the spinodal disappears, and particles fill homogeneously, which may explain the superior rate capability and long cycle life of nano-LiFePO4 cathodes. KEYWORDS: Li-ion battery, LiFePO4, phase-field model, Butler-Volmer equation, spinodal decomposition, intercalation waves. 1 Introduction. – LiFePO4-based electrochemical energy storage is one of the most promising developments for electric vehicle power systems and other high-rate applications such as power tools 2,3 and renewable energy storage. Nanoparticles of LiFePO4 have recently been used to demonstrate ultrafast battery discharge4 and high power density carbon pseudocapacitors.5 However, the -

1504.05850V4.Pdf
Compact Stars in the QCD Phase Diagram IV (CSQCD IV) September 26-30, 2014, Prerow, Germany http://www.ift.uni.wroc.pl/˜csqcdiv Entropic and enthalpic phase transitions in high energy density nuclear matter Igor Iosilevskiy1,2 1 Joint Institute for High Temperatures (Russian Academy of Sciences), Izhorskaya Str. 13/2, 125412 Moscow, Russia 2 Moscow Institute of Physics and Technology (State Research University), Dolgoprudny, 141700, Moscow Region, Russia 1 Abstract Features of Gas-Liquid (GL) and Quark-Hadron (QH) phase transitions (PT) in dense nuclear matter are under discussion in comparison with their terrestrial coun- terparts, e.g. so-called ”plasma” PT in shock-compressed hydrogen, nitrogen etc. Both, GLPT and QHPT, when being represented in widely accepted temperature – baryonic chemical potential plane, are often considered as similar, i.e. amenable to one-to-one mapping by simple scaling. It is argued that this impression is illusive and that GLPT and QHPT belong to different classes: GLPT is typical enthalpic PT (Van-der-Waals-like) while QHPT (”deconfinement-driven”) is typical entropic PT. Subdivision of 1st-order fluid-fluid phase transitions into enthalpic and entropic subclasses was proposed in [arXiv:1403.8053]. Properties of enthalpic and entropic PTs differ significantly. Entropic PT is always internal part of more general and ex- tended thermodynamic anomaly – domains with abnormal (negative) sign for the set of (usually positive) second derivatives of thermodynamic potential, e.g. Gruneizen and thermal expansion and thermal pressure coefficients etc. Negative sign of these derivatives lead to violation of standard behavior and relative order for many iso-lines in P –V plane, e.g. -
![Arxiv:2010.01933V2 [Cond-Mat.Quant-Gas] 18 Feb 2021 Tigated in Refs](https://docslib.b-cdn.net/cover/8490/arxiv-2010-01933v2-cond-mat-quant-gas-18-feb-2021-tigated-in-refs-768490.webp)
Arxiv:2010.01933V2 [Cond-Mat.Quant-Gas] 18 Feb 2021 Tigated in Refs
Finite temperature spin dynamics of a two-dimensional Bose-Bose atomic mixture Arko Roy,1, ∗ Miki Ota,1, ∗ Alessio Recati,1, 2 and Franco Dalfovo1 1INO-CNR BEC Center and Universit`adi Trento, via Sommarive 14, I-38123 Trento, Italy 2Trento Institute for Fundamental Physics and Applications, INFN, 38123 Povo, Italy We examine the role of thermal fluctuations in uniform two-dimensional binary Bose mixtures of dilute ultracold atomic gases. We use a mean-field Hartree-Fock theory to derive analytical predictions for the miscible-immiscible transition. A nontrivial result of this theory is that a fully miscible phase at T = 0 may become unstable at T 6= 0, as a consequence of a divergent behaviour in the spin susceptibility. We test this prediction by performing numerical simulations with the Stochastic (Projected) Gross-Pitaevskii equation, which includes beyond mean-field effects. We calculate the equilibrium configurations at different temperatures and interaction strengths and we simulate spin oscillations produced by a weak external perturbation. Despite some qualitative agreement, the comparison between the two theories shows that the mean-field approximation is not able to properly describe the behavior of the two-dimensional mixture near the miscible-immiscible transition, as thermal fluctuations smoothen all sharp features both in the phase diagram and in spin dynamics, except for temperature well below the critical temperature for superfluidity. I. INTRODUCTION ing the Popov theory. It is then natural to ask whether such a phase-transition also exists in 2D. The study of phase-separation in two-component clas- It is worth stressing that, in 2D Bose gases, thermal sical fluids is of paramount importance and the role of fluctuations are much more important than in 3D, as they temperature can be rather nontrivial. -

Nucleation Initiated Spinodal Decomposition in a Polymerizing System Thein Yk U University of Akron Main Campus, [email protected]
The University of Akron IdeaExchange@UAkron College of Polymer Science and Polymer Engineering 5-1996 Nucleation Initiated Spinodal Decomposition in a Polymerizing System Thein yK u University of Akron Main Campus, [email protected] Jae Hyung Lee University of Akron Main Campus Please take a moment to share how this work helps you through this survey. Your feedback will be important as we plan further development of our repository. Follow this and additional works at: http://ideaexchange.uakron.edu/polymer_ideas Part of the Polymer Science Commons Recommended Citation Kyu, Thein nda Lee, Jae Hyung, "Nucleation Initiated Spinodal Decomposition in a Polymerizing System" (1996). College of Polymer Science and Polymer Engineering. 67. http://ideaexchange.uakron.edu/polymer_ideas/67 This Article is brought to you for free and open access by IdeaExchange@UAkron, the institutional repository of The nivU ersity of Akron in Akron, Ohio, USA. It has been accepted for inclusion in College of Polymer Science and Polymer Engineering by an authorized administrator of IdeaExchange@UAkron. For more information, please contact [email protected], [email protected]. VOLUME 76, NUMBER 20 PHYSICAL REVIEW LETTERS 13MAY 1996 Nucleation Initiated Spinodal Decomposition in a Polymerizing System Thein Kyu and Jae-Hyung Lee Institute of Polymer Engineering, The University of Akron, Akron, Ohio 44325 (Received 29 September 1995) Dynamics of phase separation in a polymerizing system, consisting of carboxyl terminated polybutadiene acrylonitrile/epoxy/methylene dianiline, was investigated by means of time-resolved light scattering. The initial length scale was found to decrease for some early periods of the reaction which has been explained in the context of nucleation initiated spinodal decomposition. -

Rnase Refolding • General Features of Globular Proteins • Interior Packing
Lecture Notes - 3 7.24/7.88J/5.48J The Protein Folding and Human Disease • Reprise RNase refolding • General Features of Globular proteins • Interior Packing Rnase Refolding We can cartoon the experiments: Assume that reduced form is unfolded and populates an ensemble of unfolded states in 8M urea: [Unfolded] [Intermediate] [Native] [U] Rapidly exchanging statistical ensemble of random coils [I] ??? [N] Native fold, $ S-S bonds, 1 out of 105 possible sets of S-S bonds [Aggregated] Non-native (no enzymatic activity), non-native (scrambled) disulfide bonds. At the end of the reaction we have native state Soluble inactive – misfolded or perhaps small oligomers Precipitated: Anfinsen showed that these were S-S bonded network = he called “scrambled” Critical question is nature of the intermediates as go from here to there: If there was a sequence in which these bonds formed, and one could figure out the sequence or pathway, it might give you considerable information on the steps in the folding pathway. So certainly we need to understand native-fold, and interactions that determine it, stabilize it once formed; However this does not solve problem We will return to the question after reviewing the general anatomy of globular proteins, which will occupy most of next week. 1 General features of 3-D structures of solved proteins; Globular, Soluble proteins: A. Isolation and Crystallization The first crystals of proteins sufficiently large and ordered to diffract X-rays were prepared of the digestive enzyme pepsin: Bernal, J. D., and Dorothy Crowfoot. Nature (1934): 133, 794. This was followed by crystals of insulin, Lactalbumin, hemoglobin, and chymotrypsin: Bernal, Fankuchen, and Perutz (1938) The rise to power of the Nazis in Germany and Italy and the outbreak of war brought all these studies to a halt. -

Spinodal Lines and Equations of State: a Review
10 l Nuclear Engineering and Design 95 (1986) 297-314 297 North-Holland, Amsterdam SPINODAL LINES AND EQUATIONS OF STATE: A REVIEW John H. LIENHARD, N, SHAMSUNDAR and PaulO, BINEY * Heat Transfer/ Phase-Change Laboratory, Mechanical Engineering Department, University of Houston, Houston, TX 77004, USA The importance of knowing superheated liquid properties, and of locating the liquid spinodal line, is discussed, The measurement and prediction of the spinodal line, and the limits of isentropic pressure undershoot, are reviewed, Means are presented for formulating equations of state and fundamental equations to predict superheated liquid properties and spinodal limits, It is shown how the temperature dependence of surface tension can be used to verify p - v - T equations of state, or how this dependence can be predicted if the equation of state is known. 1. Scope methods for making simplified predictions of property information, which can be applied to the full range of Today's technology, with its emphasis on miniaturiz fluids - water, mercury, nitrogen, etc. [3-5]; and predic ing and intensifying thennal processes, steadily de tions of the depressurizations that might occur in ther mands higher heat fluxes and poses greater dangers of mohydraulic accidents. (See e.g. refs. [6,7].) sending liquids beyond their boiling points into the metastable, or superheated, state. This state poses the threat of serious thermohydraulic explosions. Yet we 2. The spinodal limit of liquid superheat know little about its thermal properties, and cannot predict process behavior after a liquid becomes super heated. Some of the practical situations that require a 2.1. The role of the equation of state in defining the spinodal line knowledge the limits of liquid superheat, and the physi cal properties of superheated liquids, include: - Thennohydraulic explosions as might occur in nuclear Fig. -

Notes on Phase Seperation Chem 130A Fall 2002 Prof
Notes on Phase Seperation Chem 130A Fall 2002 Prof. Groves Consider the process: Pure 1 + Pure 2 Æ Mixture of 1 and 2 We have previously derived the entropy of mixing for this process from statistical principles and found it to be: ∆ =− − Smix kN11ln X kN 2 ln X 2 where N1 and N2 are the number of molecules of type 1 and 2, respectively. We use k, the Boltzmann constant, since N1 and N2 are number of molecules. We could alternatively use R, the gas constant, if we chose to represent the number of molecules in terms of moles. X1 and X2 represent the mole fraction (e.g. X1 = N1 / (N1+N2)) of 1 and 2, respectively. If there are interactions between the two ∆ components, there will be a non-zero Hmix that we must consider. We can derive an expression ∆H = 2γ ∆ ∆ for Hmix using H of swapping one molecule of type 1, in pure type 1, with one molecule of type 2, in pure type 2 (see adjacent figure). We define γ as half of this ∆H of interchange. It is a differential interaction energy that tells us the energy difference between 1:1 and 2:2 interactions and 1:2 interactions. By defining 2γ = ∆H, γ represents the energy change associated with one molecule (note that two were involved in the swap). ∆ γ Now, to calculate Hmix from , we first consider 1 molecule of type 1 in the mixed system. For the moment we need not be concerned with whether or not they actually will mix, we just want ∆ to calculate Hmix as if they mix thoroughly. -

Triangulation of Analytic Molecular Surface
Masaryk University Faculty of Informatics Triangulation of analytic molecular surface Bachelor’s Thesis Radoslav Mráz Brno, Spring 2018 Masaryk University Faculty of Informatics Triangulation of analytic molecular surface Bachelor’s Thesis Radoslav Mráz Brno, Spring 2018 This is where a copy of the official signed thesis assignment and a copy of the State- ment of an Author is located in the printed version of the document. Declaration Hereby I declare that this paper is my original authorial work, which I have worked out on my own. All sources, references, and literature used or ex- cerpted during elaboration of this work are properly cited and listed in com- plete reference to the due source. Radoslav Mráz Advisor: RNDr. Adam Jurčík Ph.D. i Acknowledgements I would like to thank my advisor RNDr. Adam Jurčík Ph.D. for his consulta- tions and insights throughout writing of this thesis. Moreover I would like to thank my family for supporting me in my studies. iii Abstract The goal of this thesis is to implement an algorithm for triangulating ana- lytically represented solvent-excluded molecular surface. The first part of this thesis describes related molecular surfaces, the solvent-excluded sur- face itself and significant works in this field. The second part presents an algorithm for generating a triangular mesh of the solvent-excluded surface. The last part contains results from testing of the presented algorithm. iv Keywords molecular surface, SES, solvent-excluded surface, triangulation v Contents Introduction 1 1 Molecular surfaces 3 1.1 Van der Waals surface .......................3 1.2 Solvent-accessible surface ......................3 1.3 Solvent-excluded surface ......................4 2 Analytic representation and methods of computation of SES 7 2.1 Connoly’s definition of the SES ...................7 2.2 Reduced surface .......................... -

Phase Equilibria of Quasi-Ternary Systems Consisting of Multicomponent Polymers in a Binary Solvent Mixture IV
Polymer Journal, Vol. 18, No.4, pp 347-360 (1986) Phase Equilibria of Quasi-Ternary Systems Consisting of Multicomponent Polymers in a Binary Solvent Mixture IV. Spinodal Curve and Critical Solution Point Kenji KAMIDE and Shigenobu MATSUDA Fundamental Research Laboratory of Fiber and Fiber-Forming Polymers, Asahi Chemical Industry Company, Ltd., 11-7, Hacchonawate, Takatsuki, Osaka 569, Japan (Received October 28, 1985) ABSTRACT: An attempt was made to construct a quasi-ternary system cons1stmg of multicomponent polymers dissolved in binary solvent mixture (solvents I and 2) to derive the equations of spinodal and neutral equilibrium conditions and to clarify the effects of three thermodynamic interaction x-parameters between solvent I and solvent 2, solvent I and polymer, and solvent 2 and polymer(x 12, x13, and x23) and the weight-average degree of polymerization and the ratio of to the number-average degree of polymerization of the original polymer on the spinodal curve and the critical points (the critical concentration calculated from the above mentioned equations. The cloud point curve was also calculated indirectly from coexisting curve evaluated according to a method in our previous papers and constant (starting polymer volume fraction) line. The cross point of a constant line and a coexisting curve is a cloud point. The cloud point curve thus calculated was confirmed to coincide, as theory predicted with the spinodal curve, at the critical point. decreases with an increase in X12, x23, and and increases with an increase in x13 -
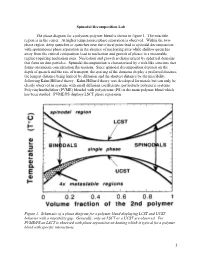
Spinodal Decomposition Lab.Pdf
Spinodal Decomposition Lab The phase diagram for a polymer-polymer blend is shown in figure 1. The miscible region is in the center. At higher temperatures phase separation is observed. Within the two- phase region, deep quenches or quenches near the critical point lead to spinodal decomposition with spontaneous phase separation in the absence of nucleating sites while shallow quenches away from the critical composition lead to nucleation and growth of phases in a metastable regime requiring nucleation sites. Nucleation and growth is characterized by spherical domains that form on dust particles. Spinodal decomposition is characterized by a web-like structure that forms on random concentration fluctuations. Since spinodal decomposition depends on the depth of quench and the rate of transport, the spacing of the domains display a preferred distance, the longest distance being limited by diffusion and the shortest distance by the miscibility following Kahn-Hilliard theory. Kahn-Hilliard theory was developed for metals but can only be clearly observed in systems with small diffusion coefficients, particularly polymeric systems. Polyvinylmethylether (PVME) blended with polystyrene (PS) is the main polymer blend which has been studied. PVME/PS displays LSCT phase separation. Figure 1. Schematic of a phase diagram for a polymer blend displaying LCST and UCST behavior with a miscibility gap. Generally, only an LSCT or a UCST are observed. For PVME/PS an LSCT is observed with phase separation on heating which is typical for a polymer blend with specific interactions. 1 ri is the degree of polymerization of the polymer component “i”, fi is the volume fraction of component “i”.