Antitumor Activity of Mir-34A in Peritoneal Mesothelioma Relies on C-MET and AXL Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malignant Peritoneal Mesothelioma: Clinical Aspects, and Therapeutic Perspectives
REVIEW ARTICLE Annals of Gastroenterology (2018) 31, 1-11 Malignant peritoneal mesothelioma: clinical aspects, and therapeutic perspectives Stergios Boussiosa, Michele Moschettab, Afroditi Karathanasia, Alexandros K. Tsiourisc, Foivos S. Kanellosc, Konstantina Tatsid, Konstantinos H. Katsanose, Dimitrios K. Christodouloue Medway NHS Foundation Trust, Kent, UK; Sarah Cannon Research Institute, London, UK; University of Ioannina, Greece; General Hospital G. Hatzikosta, Ioannina, Greece Abstract Malignant peritoneal mesothelioma (MPM) is a rare disease with a wide clinical spectrum. It arises from the peritoneal lining and commonly presents with diffuse, extensive spread throughout the abdomen and, more rarely, metastatic spread beyond the abdominal cavity. Computed tomography, magnetic resonance imaging and positron-emission tomography are important diagnostic tools used for the preoperative staging of MPM. The definitive diagnosis is based on histopathological analysis, mainly via immunohistochemistry. In this regard, paired- box gene 8 negativity represents a useful diagnostic biomarker for differentiating MPM from ovarian carcinoma. In addition, BRCA1-associated protein-1 (BAP1) loss is specific to MPM and allows it to be distinguished from both benign mesothelial lesions and ovarian serous tumors. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become an increasingly important therapeutic approach, while systemic therapies are still being developed. Histology, Ki-67, completeness of cytoreduction, -

Mesothelin's Role As a Biomarker and Therapeutic Target for Malignant
cancers Review Hitting the Bull’s-Eye: Mesothelin’s Role as a Biomarker and Therapeutic Target for Malignant Pleural Mesothelioma Dannel Yeo 1,2,3 , Laura Castelletti 1,2,3 , Nico van Zandwijk 2,3,4 and John E. J. Rasko 1,2,3,5,* 1 Li Ka Shing Cell & Gene Therapy Program, The University of Sydney, Camperdown, NSW 2050, Australia; [email protected] (D.Y.); [email protected] (L.C.) 2 Faculty of Medicine and Health, The University of Sydney, Camperdown, NSW 2050, Australia; [email protected] 3 Cell and Molecular Therapies, Royal Prince Alfred Hospital, Sydney Local Health District (SLHD), Camperdown, NSW 2050, Australia 4 Concord Repatriation General Hospital, Sydney Local Health District (SLHD), Concord, NSW 2139, Australia 5 Gene and Stem Cell Therapy Program, Centenary Institute, The University of Sydney, Camperdown, NSW 2050, Australia * Correspondence: [email protected]; Tel.: +61-295656160 Simple Summary: Mesothelioma is a deadly disease with a dismal prognosis. Since its discovery, mesothelin, a cell surface protein, has been a promising biomarker and therapeutic target due to its overexpression in mesothelioma and limited expression in normal cells. This review summarizes the clinical studies that have examined mesothelin as a biomarker and therapeutic target in mesothelioma and explores future perspectives in its role to improve patient management. Abstract: Malignant pleural mesothelioma (MPM) is an aggressive cancer with limited treatment options and poor prognosis. MPM originates from the mesothelial lining of the pleura. Mesothelin Citation: Yeo, D.; Castelletti, L.; van (MSLN) is a glycoprotein expressed at low levels in normal tissues and at high levels in MPM. -

UEMS 2020.11 Syllabus of the ETR in Rare Adult Cancers
UNION EUROPÉENNE DES MÉDECINS SPÉCIALISTES EUROPEAN UNION OF MEDICAL SPECIALISTS Association internationale sans but lucratif International non-profit organisation RUE DE L’INDUSTRIE, 24 T +32 2 649 51 64 BE- 1040 BRUSSELS F +32 2 640 37 30 www.uems.eu [email protected] UEMS 2020.11 Syllabus for residents and trainees in Rare Adult Solid Cancers The basic goal of this syllabus is to provide an understanding between the instructor and trainee so there is minimal confusion in the topics, with clear expectations. It is not a classical syllabus as it contains descriptions from different areas, but it still summarizes major and specific topics that should be covered during the training course of a resident. This syllabus is intended as supporting reference material, and the precise content and priorities of training may vary in different training institutions. The syllabus can also be modified to reflect each instructor's teaching philosophy towards the trainees. 1. There are scientific publications, web pages, and conference materials available online that could be used for educational purposes for various types of rare adult solid cancers. This is a comprehensive summary of them. 2. There are significant differences in the number of available scientific publications and reviews for different rare adult solid cancers. Some, like sarcomas, have a very robust literature, while others have been sparsely researched and consequently the availability of study materials is quite poor. 3. These differences also apply to life events and natural history. In the list of the EU CE accredited events there is a strong underrepresentation for some types of rare adult solid cancers. -

Ct Findings of Hypervascular Malignant Peritoneal Mesothelioma
Compurerized Radial. Vol. I I, No. 2, pp. 91-94, 1987 0730-4862/87 53.00 + 0.00 Printed in the U.S.A. All rights reserved Copyright 8 1987 Pergamon Journals Ltd CT FINDINGS OF HYPERVASCULAR MALIGNANT PERITONEAL MESOTHELIOMA DEBORAH S. GRANKE,* JAMES H. ELLIS and BRUCE D. RICHMOND Radiology Service (114) Veterans Administration Medical Center and Department of Radiology. University of Michigan Medical School, Ann Arbor, MI 48105. U.S.A. (Received 19 June 1986; in revised form 21 October 1986; received for publicatiorr 6 November 1986) Abstract-A case of peritoneal mesothelioma is presented in which CT demonstrated abnormal regions of increased vascularity in the omentum corresponding to hypervascular omental lesions shown by angiography. This CT appearance has not been described in prior reports of CT in peritoneal mesothelioma. Mesothelioma. peritoneal Angiography Computed tomography INTRODUCTION Reports of computed tomography (CT) in mesothelioma describe peritoneal involvement that may be extensive, with confluent tumor in layers, masses, and/or nodules and mesenteric infiltration [l, 21. A recent report of anteriography in peritoneal mesothelioma described three cases of mildly to moderately hypervascular omental lesions without arteriovenous shunting; however, the one CT scan performed was nondiagnostic [3]. We report a case of peritoneal mesothelioma where CT demon- strated abnormal regions of increased vascularity in the omentum corresponding to the hypervascular omental lesions shown by angiography. CASE REPORT A 54-year-old white male presented with a 3-month history of insidious onset of diffuse abdominal tenderness, early satiety, abdominal bloating, and crampy abdominal pain. His physical exam was unremarkable, and routine laboratory tests, sigmoidoscopy, and barium enema were normal. -

Desmoplastic Small Round Cell Tumor of the Abdomen: a Case Report and Literature Review of Therapeutic Options
Vol.4, No.4, 207-211 (2012) Health http://dx.doi.org/10.4236/health.2012.44031 Desmoplastic small round cell tumor of the abdomen: A case report and literature review of therapeutic options Hafida Benhammane1*, Leila Chbani2, Abdelmalek Ousadden3, Ouadii Mouquit3, Siham Tizniti4, Afaf Riffi Amarti2, Nouafal Mellas1, Omar El Mesbahi1 1Department of Medical Oncology, Hassan II University Hospital, Fez, Morocco; *Corresponding Author: [email protected] 2Department of Pathology, Hassan II University Hospital, Fez, Morocco 3Department of General Surgery, Hassan II University Hospital, Fez, Morocco 4Department of Radiology, Hassan II University Hospital, Fez, Morocco Received 22 December 2011; revised 18 January 2012; accepted 6 February 2012 ABSTRACT rent therapeutic options include multiagent chemothe- rapy and aggressive surgical debulking and radiotherapy Desmoplastic small round cell tumor (DSRCT) is [4,5]. The addition of hyperthermic intra-peritoneal che- a rare and highly aggressive variety of sarcoma motherapy in the multimodal approach has been reported arising typically from abdominal or pelvic peri- in very few cases but no effect on survival has been clearly toneum. Diagnosis and treatment approaches of demonstrated [6]. this entity are complex and require a skilled, ex- The prognosis of this disease is poor with a Median perienced, multidisciplinary team. Authors re- survival of 17 months approximately [7]. port their experience with a case of an intra-ab- We report a case of an intra-abdominal DSRCT in a 37 dominal DSRCT arising in a 37-year-old young -year-old young man who was treated with combination man in order to discuss the clinico-pathological chemotherapy and surgery. -

"Gastrointestinal Tract Pathology"
IIC,J CALIFORNIA TUMOR TISSUE REGISTRY "GASTROINTESTINAL TRACT PATHOLOGY" Study Cases, Subscription A March 2000 California Tumor Tissue Registry c/o: De1mrtment of Pathology and Human Anatomy Loma Linda Univcr.;ily School ofMcd.icine 11021 Campus Avenue, AH 335 Lomn Linda, California 92350 (909) 558-4788 FAX: (909) 558·0188 E-mail: [email protected] Case oftbe Month: www.llu.edu/Uu/cttr/cotm Target audience: Practicing pathologists and pathology residents. Goal: To acquairu the participam with the hiswlogic featu res of a variety of benign and malignant neoplasms and tumor-l ike conditions. Objectives: n1e participant will be able to recognize morphologic features ofa variety of benign and malignam neoplasms and tWllOr-like conditions and relate those processes to pertinent references in d1e medical literature. Educational methods and media: Review of representative glass slides v.ith associated histories. Feedback on consensus diagnoses lt·om participating pathologists. Listing of selected references from the medical literature. Principal faculty: Weldon K. Bullock, Ml) Donald R. Chase, MD CME Credit: Lorna Li.nda University School of Medicine designates this continuing medical education·activity for up to 2 hours of Category I of the Physician's Recogn ition Award oft he· American Medical Association. CME credit is offered for d1e subscription year only. Accreditation: Loma Linda University School of Medicine is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians. Contributor: James A. Henry, M.D. Case No. 1 - March 2000 Woodbridge, VA Tissue from: Terminal ileum Accession #28502 Clinical Abstract: This 37-year-old black female presented with several weeks' history of right lower quadrant abdominal pain radiating to the right side of the back and right inguinal area. -

Localized Biphasic Malignant Peritoneal Mesothelioma with Rhabdoid Features Involving the Liver: Case Report and Review of the Literature
Hindawi Case Reports in Pathology Volume 2019, Article ID 2732674, 7 pages https://doi.org/10.1155/2019/2732674 Case Report Localized Biphasic Malignant Peritoneal Mesothelioma with Rhabdoid Features Involving the Liver: Case Report and Review of the Literature Dalal Hassan and Saverio Ligato Department of Pathology and Laboratory Medicine, Hartford Hospital, Hartford, CT, USA Correspondence should be addressed to Dalal Hassan; [email protected] Received 24 April 2019; Accepted 15 July 2019; Published 28 July 2019 Academic Editor: Fatemeh Mahjoub Copyright © 2019 Dalal Hassan and Saverio Ligato. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction. Localized malignant mesotheliomas, defned as sharply circumscribed tumors of the serosal membrane with the microscopic appearance of difuse malignant mesothelioma, are rare tumors; their behavior and prognosis are uncertain. Intrahepatic mesotheliomas are postulated to arise from mesothelial cells of Glisson’s capsule. Case Presentation.A69-year-old female with no history of asbestos exposure presented with a one-month history of increasing abdominal pain associated with constitutional symptoms. Computerized Tomography (CT) scan of the abdomen and pelvis revealed a sizable sof tissue mass within the right paracolic gutter, abutting the inferior hepatic margin, the lateral abdominal wall, and descending colon. Ultrasound- guided biopsy of the mass suggested a poorly diferentiated hepatocellular carcinoma. Tere was no disease elsewhere on PET scan. Surgical resection of the mass was performed. Pathological assessment suggested the tumor to be arising from the liver with invasion of the liver, abdominal wall musculature, and the adventitial surface of the ascending colon. -

Rare Epithelial Tumours of the Digestive System 17 532
RARE EPITHELIAL TUMOURS OF THE 16 % OF DIGESTIVE SYSTEM TUMOURS ARE RARE DIGESTIVE SYSTEM EPITHELIAL TUMOURS 2 262 EPITHELIAL TUMOURS OF OESOPHAGUS 81 % OF RARE EPITHELIAL TUMOURS 271 RARE EPITHELIAL TUMOURS 1 OUT OF ALL TUMOURS OF STOMACH IN EACH SITE 696 EPITHELIAL TUMOURS OF SMALL INTESTINE 56 87 RARE EPITHELIAL TUMOURS <1 INCIDENCE OF COLON 17 532 97 RARE EPITHELIAL TUMOURS OF RECTUM 1 ESTIMATED NEW CASES 1 143 EPITHELIAL TUMOURS OF ANAL CANAL 97 ITALY, 2015 RARE EPITHELIAL TUMOURS 71 OF PANCREAS 1 EPITHELIAL TUMOURS OF LIVER 7 291 AND INTRAHEPATIC BILE TRACT 53 EPITHELIAL TUMOURS OF GALLBLADDER 5 483 AND EXTRAHEPATIC BILIARY TRACT 99 132 MESOTHELIOMA OF PERITONEUM PREVALENCE SURVIVAL 43 452 100% ESTIMATED PREVALENT CASES ITALY, 2010 50% 20% 0 1 5 YEARS AFTER DIAGNOSIS SOURCE: AIRTUM. ITALIAN CANCER FIGURES–REPORT 2015 RARE EPITHELIAL TUMOURS OF THE DIGESTIVE SYSTEM I tumori in Italia • Rapporto AIRTUM 2015 INCIDENCE RARE EPITHELIAL TUMOURS OF THE DIGESTIVE SYSTEM. Crude incidence (rate per 100,000/year) and 95% confidence interval (95% CI), observed cases and proportion of rare cancers on all (common + rare) cancers by site. Rates with 95% CI by sex and age. Estimated new cases at 2015 in Italy. AIRTUM POOL (period of diagnosis 2000-2010) ITALY SEX AGE MALE FEMALE 0-54 yrs 55-64 yrs 65+ yrs ESTIMATED NEW CASES RATE 95% CI RATE 95% CI RATE 95% CI RATE 95% CI RATE 95% CI RATE 95% CI 2015 OBSERVED CASES OBSERVED (No.) EPITHELIAL RARE SITE BY CANCERS (%) RARE EPITHELIAL TUMOURS 26.11 25.89-26.32 57 891 16% 32.11 31.78-32.45 20.48 20.22-20.74 -
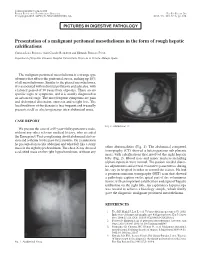
Presentation of a Malignant Peritoneal Mesothelioma in the Form of Rough
1130-0108/2017/109/5/374 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS REV ESP ENFERM DIG © Copyright 2017. SEPD y © ARÁN EDICIONES, S.L. 2017, Vol. 109, N.º 5, pp. 374 PICTURES IN DIGESTIVE PATHOLOGY Presentation of a malignant peritoneal mesothelioma in the form of rough hepatic calcifications Carmen Lara-Romero, Aida Casado-Bernabéu and Eduardo Romero-Pérez Department of Digestive Diseases. Hospital Universitario Virgen de la Victoria. Málaga, Spain The malignant peritoneal mesothelioma is a strange type of tumor that affects the peritoneal serosa, making up 10% of all mesotheliomas. Similar to the pleural mesothelioma, it is associated with industrial pollutants and asbestos, with a latency period of 30 years from exposure. There are no specific signs or symptoms, and it is usually diagnosed at an advanced stage. The most frequent symptoms are pain and abdominal distension, anorexia and weight loss. The localized form of the disease is less frequent and it usually presents itself as a heterogeneous intra-abdominal mass. CASE REPORT Fig. 2. Abdominal TC. We present the case of a 60-year-old hypertensive male, without any other relevant medical history, who attended the Emergency Unit complaining about abdominal disten- sion and asthenia for the past three months. On examination he presented an ascitic abdomen and what felt like a stony mass in the right hypochondrium. The chest X-ray showed other abnormalities (Fig. 1). The abdominal computed a calcified mass on the right hypochondrium, without any tomography (CT) showed a heterogeneous sub-phrenic mass, with calcifications that involved the right hepatic lobe (Fig. 2). Blood tests and tumor markers including alphafetoprotein were normal. -

Malignant Peritoneal Mesothelioma with Lymph Node Metastasis
Takehara et al. World Journal of Surgical Oncology 2014, 12:112 http://www.wjso.com/content/12/1/112 WORLD JOURNAL OF SURGICAL ONCOLOGY CASE REPORT Open Access Malignant peritoneal mesothelioma with lymph node metastasis that originated in the transverse colon Yusuke Takehara*, Shungo Endo, Yuichi Mori, Kenta Nakahara, Daisuke Takayanagi, Shoji Shimada, Tomokatsu Omoto, Chiyo Maeda, Shumpei Mukai, Eiji Hidaka, Fumio Ishida, Jun-ichi Tanaka and Shin-ei Kudo Abstract Background: We report an extremely rare case of resection of localized biphasic malignant peritoneal mesothelioma of the transverse colon. Case report: Computed tomography and magnetic resonance imaging in a 72-year-old man showed a tumor with enhanced borders consistent with the transverse colon. Colonoscopy showed ulcerative lesions in the transverse colon, but histological examination showed no malignancy. A gastrointestinal stromal tumor was strongly suspected, so an extended right hemicolectomy was performed. Histopathological examination showed that the tumor was a localized malignant peritoneal mesothelioma of the transverse colon. The patient did not receive postoperative chemotherapy and died 18 months after surgery. Conclusions: The number of patients with malignant mesotheliomas is predicted to increase in the future both in Japan and in western countries. We report this case due to its probable usefulness in future studies pertaining to the diagnosis and treatment of malignant mesotheliomas. Keywords: Malignant peritoneal mesothelioma, localized mesothelioma, transverse colon Background admission, the patient noticed a mass in his upper ab- Malignant mesotheliomas are rare tumors that reportedly domen; two months before admission, he experienced account for 0.2% of all malignant tumors [1,2]. Malignant abdominal pain. -

Dr-John-Allendorf-CV.Pdf
John D. Allendorf, M.D., F.A.C.S. 120 Mineola Blvd. Mineola, NY 11501 Education Columbia University College of Physicians and Surgeons, M.D., May 1997 Johns Hopkins University, B.A., Biology, May 1992 Postgraduate Training Residency: General Surgery, New York Presbyterian Hospital – Columbia Campus, New York, NY July 1997 – June 2002 Fellowship: Liver Transplantation and Hepatobiliary Surgery, New York Presbyterian Hospital – Columbia Campus, New York, NY July 2002 – June 2003 Licensing Medicine and Surgery, New York State License #212487, 10/29/98 Board Certification American Board of Surgery 9/17/03 Recertified American Board of Surgery 12/6/12 (expires 7/1/24) Faculty Appointments Associate Professor of Clinical Surgery, SUNY Stony Brook 2015- present Assistant Professor of Surgery, Columbia University 2003 – 2013 Instructor in Clinical Surgery, Columbia University 2002 – 2003 Columbia University Positions Advisory Dean of Student Affairs, 2003 – 2005 Course Director, Gross Anatomy Correlation Clinics 2004 – 2007 Program Director, Endocrine Surgery Fellowship, 2009 – 2013 Director, Pancreatic Cyst Surveillance Program, 2010 – 2013 Hospital Appointments Vice Chair, Department of Surgery, Winthrop University Hospital, 2013 to present Chief, Division of Surgical Oncology, Winthrop University Hospital, 2013 to present Director, Endocrine Surgery, Winthrop University Hospital, 2013 to present 1 Director, Pancreas Center, Winthrop University Hospital, 2013 to present Cancer Liaison Physician to the ACS Commission on Cancer, 2013 to 2018 -

TCR² Therapeutics Receives FDA Orphan Drug Designation for Gavo-Cel for the Treatment of Cholangiocarcinoma
TCR² Therapeutics Receives FDA Orphan Drug Designation for Gavo-cel for the Treatment of Cholangiocarcinoma September 2, 2021 CAMBRIDGE, Mass., Sept. 02, 2021 (GLOBE NEWSWIRE) -- TCR2 Therapeutics Inc. (Nasdaq: TCRR), a clinical-stage cell therapy company with a pipeline of novel T cell therapies for patients suffering from cancer, today announced that the U.S. Food and Drug Administration (FDA) has granted the Company Orphan Drug Designation (ODD) to gavo-cel for the treatment of cholangiocarcinoma. New clinical data from the dose escalation portion of the Phase 1/2 clinical trial of gavo-cel in patients with treatment refractory mesothelin-expressing solid tumors will be highlighted as part of an oral presentation at the European Society for Medical Oncology on September 17 at 14:20 CEST (8:20AM EST) and will include data for gavo-cel in malignant mesothelioma, ovarian cancer and cholangiocarcinoma. About Mesothelin-Expressing Solid Tumors Mesothelin is a cell-surface glycoprotein highly expressed in a wide range of solid tumors, including malignant pleural/peritoneal mesothelioma, ovarian cancer, cholangiocarcinoma, breast cancer, pancreatic cancer and others. Overexpression of mesothelin is associated with poorer prognosis in some cancers due to its active role in both malignant transformation and tumor aggressiveness by promoting cancer cell proliferation, invasion, and metastasis. Of the wide range of solid tumors expressing mesothelin, non-small cell lung cancer, ovarian cancer, mesothelioma and cholangiocarcinoma represent a patient population up to 80,000 annually in the United States alone. About TCR2 Therapeutics TCR2 Therapeutics Inc. is a clinical-stage cell therapy company developing a pipeline of novel T cell therapies for patients suffering from cancer.