Salinity Tolerances and Use of Saline Environments by Freshwater Turtles: Implications of Sea Level Rise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laws of Malaysia
LAWS OF MALAYSIA ONLINE VERSION OF UPDATED TEXT OF REPRINT Act 716 WILDLIFE CONSERVATION ACT 2010 As at 1 December 2014 2 WILDLIFE CONSERVATION ACT 2010 Date of Royal Assent … … 21 October 2010 Date of publication in the Gazette … … … 4 November 2010 Latest amendment made by P.U.(A)108/2014 which came into operation on ... ... ... ... … … … … 18 April 2014 3 LAWS OF MALAYSIA Act 716 WILDLIFE CONSERVATION ACT 2010 ARRANGEMENT OF SECTIONS PART I PRELIMINARY Section 1. Short title and commencement 2. Application 3. Interpretation PART II APPOINTMENT OF OFFICERS, ETC. 4. Appointment of officers, etc. 5. Delegation of powers 6. Power of Minister to give directions 7. Power of the Director General to issue orders 8. Carrying and use of arms PART III LICENSING PROVISIONS Chapter 1 Requirement for licence, etc. 9. Requirement for licence 4 Laws of Malaysia ACT 716 Section 10. Requirement for permit 11. Requirement for special permit Chapter 2 Application for licence, etc. 12. Application for licence, etc. 13. Additional information or document 14. Grant of licence, etc. 15. Power to impose additional conditions and to vary or revoke conditions 16. Validity of licence, etc. 17. Carrying or displaying licence, etc. 18. Change of particulars 19. Loss of licence, etc. 20. Replacement of licence, etc. 21. Assignment of licence, etc. 22. Return of licence, etc., upon expiry 23. Suspension or revocation of licence, etc. 24. Licence, etc., to be void 25. Appeals Chapter 3 Miscellaneous 26. Hunting by means of shooting 27. No licence during close season 28. Prerequisites to operate zoo, etc. 29. Prohibition of possessing, etc., snares 30. -

USF Board of Trustees ( March 7, 2013)
Agenda item: (to be completed by Board staff) USF Board of Trustees ( March 7, 2013) Issue: Proposed Ph.D. in Integrative Biology ________________________________________________________________ Proposed action: New Degree Program Approval ________________________________________________________________ Background information: This application for a new Ph.D is driven by a recent reorganization of the Department of Biology. The reorganization began in 2006 and was completed in 2009. The reorganization of the Department of Biology, in part, reflected the enormity of the biological sciences, and in part, different research perspectives and directions taken by the faculty in each of the respective areas of biology. Part of the reorganization was to replace the original Ph.D. in Biology with two new doctoral degrees that better serve the needs of the State and our current graduate students by enabling greater focus of the research performed to earn the Ph.D. The well-established and highly productive faculty attracts students to the Tampa Campus from all over the United States as well as from foreign countries. The resources to support the two Ph.D. programs have already been established in the Department of Biology and are sufficient to support the two new degree programs. The reorganization created two new departments; the Department of Cell Biology, Microbiology, and Molecular Biology (CMMB) and the Department of Integrative Biology (IB). This proposal addresses the creation of a new Ph.D., in Integrative Biology offered by the Department of Integrative Biology (CIP Code 26.1399). The name of the Department, Integrative Biology, reflects the belief that the study of biological processes and systems can best be accomplished by the incorporation of numerous integrated approaches Strategic Goal(s) Item Supports: The proposed program directly supports the following: Goal 1 and Goal 2 Workgroup Review: ACE March 7, 2013 Supporting Documentation: See Complete Proposal below Prepared by: Dr. -

Phylogenetic Relationships of the Asian Box Turtles of the Genus Cuora Sensu Lato (Reptilia: Bataguridae) Inferred from Mitochondrial DNA Sequences
ZOOLOGICAL SCIENCE 19: 1305–1312 (2002) 2002 Zoological Society of Japan Phylogenetic Relationships of the Asian Box Turtles of the Genus Cuora sensu lato (Reptilia: Bataguridae) Inferred from Mitochondrial DNA Sequences Masanao Honda1*†, Yuichirou Yasukawa1, Ren Hirayama2 and Hidetoshi Ota1 1Tropical Biosphere Research Center, University of the Ryukyus, Nishihara, Okinawa 903-0213, Japan 2Faculty of Information, Teikyo Heisei University, Ichihara, Chiba 290-0193, Japan ABSTRACT—Phylogenetic relationships of the genus Cuora sensu lato (Cuora sensu stricto and Cisto- clemmys) and other testudinoid genera were inferred from variations in 882 base positions of mitochondrial 12S and 16S rRNA genes. Results yielded a robust support to the monophyly of a group (Cuora group) consisting of Cuora sensu lato and the monotypic Pyxidea. Within the Cuora group, the continental Cuora (sensu stricto) and the two subspecies of Ci. flavomarginata constituted two well-supported monophyletic groups. Distinctly small interspecific genetic distances in the former groups suggested that in the continent speciations in Cuora took place much later than the primary divergences in the Cuora group, or speciations in other related genera, such as Mauremys. Our analyses failed to provide a substantial support to the monophyly of any other combinations of taxa within the Cuora group, including Cuora in broad and strict senses, and Cistoclemmys as consisting of Ci. galbinifrons and Ci. flavomarginata. Besides these, our results also suggested the non-monophyly for the Batagurinae and the Geoemydinae, and sister relation- ships of the Bataguridae with Testudinidae rather than with the Emydidae. Key words: Bataguridae, Geoemydinae, Cuora, Cistoclemmys, Pyxidea Cu. amboinensis), Cyclemys Bell, 1834 (type species: Cy. -

A New Xinjiangchelyid Turtle from the Middle Jurassic of Xinjiang, China and the Evolution of the Basipterygoid Process in Mesozoic Turtles Rabi Et Al
A new xinjiangchelyid turtle from the Middle Jurassic of Xinjiang, China and the evolution of the basipterygoid process in Mesozoic turtles Rabi et al. Rabi et al. BMC Evolutionary Biology 2013, 13:203 http://www.biomedcentral.com/1471-2148/13/203 Rabi et al. BMC Evolutionary Biology 2013, 13:203 http://www.biomedcentral.com/1471-2148/13/203 RESEARCH ARTICLE Open Access A new xinjiangchelyid turtle from the Middle Jurassic of Xinjiang, China and the evolution of the basipterygoid process in Mesozoic turtles Márton Rabi1,2*, Chang-Fu Zhou3, Oliver Wings4, Sun Ge3 and Walter G Joyce1,5 Abstract Background: Most turtles from the Middle and Late Jurassic of Asia are referred to the newly defined clade Xinjiangchelyidae, a group of mostly shell-based, generalized, small to mid-sized aquatic froms that are widely considered to represent the stem lineage of Cryptodira. Xinjiangchelyids provide us with great insights into the plesiomorphic anatomy of crown-cryptodires, the most diverse group of living turtles, and they are particularly relevant for understanding the origin and early divergence of the primary clades of extant turtles. Results: Exceptionally complete new xinjiangchelyid material from the ?Qigu Formation of the Turpan Basin (Xinjiang Autonomous Province, China) provides new insights into the anatomy of this group and is assigned to Xinjiangchelys wusu n. sp. A phylogenetic analysis places Xinjiangchelys wusu n. sp. in a monophyletic polytomy with other xinjiangchelyids, including Xinjiangchelys junggarensis, X. radiplicatoides, X. levensis and X. latiens. However, the analysis supports the unorthodox, though tentative placement of xinjiangchelyids and sinemydids outside of crown-group Testudines. A particularly interesting new observation is that the skull of this xinjiangchelyid retains such primitive features as a reduced interpterygoid vacuity and basipterygoid processes. -

Carettochelys Insculpta) in the KIKORI REGION, PAPUA NEW GUINEA
NESTING ECOLOGY, HARVEST AND CONSERVATION OF THE PIG-NOSED TURTLE (Carettochelys insculpta) IN THE KIKORI REGION, PAPUA NEW GUINEA by CARLA CAMILO EISEMBERG DE ALVARENGA B.Sc. (Federal University of Minas Gerais – UFMG) (2004) M.Sc. (National Institute for Amazon Research) (2006) Institute for Applied Ecology University of Canberra Australia A thesis submitted in fulfilment of the requirements of the Degree of Doctor of Philosophy at the University of Canberra. October 2010 Certificate of Authorship of Thesis Except where clearly acknowledged in footnotes, quotations and the bibliography, I certify that I am the sole author of the thesis submitted today, entitled Nesting ecology, harvest and conservation of the Pig-nosed turtle (Carettochelys insculpta) in the Kikori region, Papua New Guinea I further certify that to the best of my knowledge the thesis contains no material previously published or written by another person except where due reference is made in the text of the thesis. The material in the thesis has not been the basis of an award of any other degree or diploma.The thesis complies with University requirements for a thesis as set out in Gold Book Part 7: Examination of Higher Degree by Research Theses Policy, Schedule Two (S2). …………………………………………… Signature of Candidate .......................................................................... Signature of chair of the supervisory panel ………15-Mar-11…………………….. Date Copyright This thesis (© by Carla C. Eisemberg, 2010) may be freely copied or distributed for private and/or commercial use and study. However, no part of this thesis or the information herein may be included in a publication or referred to in a publication without the written consent of Carla C. -

Movement and Habitat Use of Australias Largest Snakenecked Turtle
bs_bs_bannerJournal of Zoology Journal of Zoology. Print ISSN 0952-8369 Movement and habitat use of Australia’s largest snake-necked turtle: implications for water management D. S. Bower1*, M. Hutchinson2 & A. Georges1 1 Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia 2 South Australian Museum, Adelaide, SA, Australia Keywords Abstract freshwater; radio-telemetry; home range; weir; tortoise; river; sex differences. Hydrological regimes strongly influence ecological processes in river basins. Yet, the impacts of management regimes are unknown for many freshwater taxa in Correspondence highly regulated rivers. We used radio-telemetry to monitor the movement and Deborah S. Bower, School of Environmental activity of broad-shelled river turtles Chelodina expansa to infer the impact of and Life Sciences, University of Newcastle, current water management practices on turtles in Australia’s most regulated river Callaghan, NSW 2308, Australia. – the Murray River. We radio-tracked C. expansa to (1) measure the range span Tel: +61 02 49212045; and examine the effect of sex, size and habitat type on turtle movement, and (2) Fax: +61 02 49 216923 examine habitat use within the river channel and its associated backwaters. C. Email: [email protected] expansa occupied all macro habitats in the river (main channel, backwater, swamp and connecting inlets). Within these habitats, females occupied discrete home *Present address: School of Environmental ranges, whereas males moved up to 25 km. The extensive movement of male and Life Sciences, University of Newcastle, turtles suggests that weirs and other aquatic barriers may interfere with movement Callaghan, NSW, 2308, Australia. and dispersal. Turtles regularly move between backwaters and the main river channel, which highlights the likely disturbance from backwater detachment, a Editor: Virginia Hayssen water saving practice in the lower Murray River. -

Zootaxa, a New Subspecies of Batagur Affinis (Cantor, 1847), One of The
Zootaxa 2233: 57–68 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) A new subspecies of Batagur affinis (Cantor, 1847), one of the world’s most critically endangered chelonians (Testudines: Geoemydidae) PETER PRASCHAG1, ROHAN HOLLOWAY2, ARTHUR GEORGES2, MARTIN PÄCKERT3, ANNA K. HUNDSDÖRFER3 & UWE FRITZ3,4 1The Turtle Conservancy, Behler Chelonian Institute, P.O. Box 1289, Ojai, CA 93024, USA 2Institute of Applied Ecology, Research Group, University of Canberra, Canberra 2601, Australia 3Museum of Zoology, Senckenberg Dresden, A.B. Meyer Building, D-01109 Dresden, Germany 4Corresponding author. E-mail: [email protected] Abstract Estuarine Batagur are among the most critically endangered chelonian species. We assess the taxonomic status of the recently discovered Cambodian relic population of Batagur by phylogenetic analyses of three mitochondrial (2096 bp) and three nuclear DNA fragments (1909 bp) using sequences from all other Batagur species and selected allied geoemydids. Furthermore, we calculated haplotype networks of the mitochondrial cytochrome b gene for Cambodian terrapins, B. affinis, B. baska, and B. kachuga and compare external morphology of estuarine Batagur populations. Genetically, Cambodian Batagur are closely related with, but distinct from B. affinis from Sumatra and the west coast of the Malay Peninsula. Morphologically, Cambodian Batagur resemble the distinctive B. affinis populations from the eastern Malay Peninsula that were not available for genetic study. We suggest that the Batagur populations from the eastern Malay Peninsula and Cambodia represent a new subspecies of B. affinis that once was distributed in estuaries surrounding the Gulf of Thailand (Batagur affinis edwardmolli subsp. -
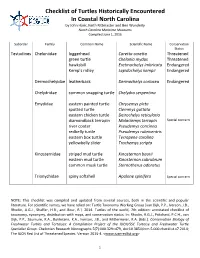
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

The Conservation Biology of Tortoises
The Conservation Biology of Tortoises Edited by Ian R. Swingland and Michael W. Klemens IUCN/SSC Tortoise and Freshwater Turtle Specialist Group and The Durrell Institute of Conservation and Ecology Occasional Papers of the IUCN Species Survival Commission (SSC) No. 5 IUCN—The World Conservation Union IUCN Species Survival Commission Role of the SSC 3. To cooperate with the World Conservation Monitoring Centre (WCMC) The Species Survival Commission (SSC) is IUCN's primary source of the in developing and evaluating a data base on the status of and trade in wild scientific and technical information required for the maintenance of biological flora and fauna, and to provide policy guidance to WCMC. diversity through the conservation of endangered and vulnerable species of 4. To provide advice, information, and expertise to the Secretariat of the fauna and flora, whilst recommending and promoting measures for their con- Convention on International Trade in Endangered Species of Wild Fauna servation, and for the management of other species of conservation concern. and Flora (CITES) and other international agreements affecting conser- Its objective is to mobilize action to prevent the extinction of species, sub- vation of species or biological diversity. species, and discrete populations of fauna and flora, thereby not only maintain- 5. To carry out specific tasks on behalf of the Union, including: ing biological diversity but improving the status of endangered and vulnerable species. • coordination of a programme of activities for the conservation of biological diversity within the framework of the IUCN Conserva- tion Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitor- 1. -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -

Membros Da Comissão Julgadora Da Dissertação
UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada Ribeirão Preto - SP 2021 UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada. Orientador: Prof. Dr. Max Cardoso Langer Ribeirão Preto - SP 2021 Autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico, para fins de estudo e pesquisa, desde que citada a fonte. I authorise the reproduction and total or partial disclosure of this work, via any conventional or electronic medium, for aims of study and research, with the condition that the source is cited. FICHA CATALOGRÁFICA Hermanson, Guilherme Evolution of the skull shape in extinct and extant turtles, 2021. 132 páginas. Dissertação de Mestrado, apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto/USP – Área de concentração: Biologia Comparada. -

Distribution and Population Status of the Narrow-Headed Softshell Turtle Chitra Spp
The Natural History Journal of Chulalongkorn University 5(1): 31-42, May 2005 ©2005 by Chulalongkorn University Distribution and Population Status of the Narrow-Headed Softshell Turtle Chitra spp. in Thailand ∗ WACHIRA KITIMASAK1,2, , KUMTHORN THIRAKHUPT1, SITDHI BOONYARATPALIN3 AND DON L. MOLL4 1Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, THAILAND 2Kanchanaburi Inland Fisheries Research and Development Center, Tha Muang, Kanchanaburi Province 71110, THAILAND 3Department of Fisheries, Kasetklang, Chatuchak, Bangkok 10900, THAILAND 4Department of Biology, Southwest Missouri State University, Springfield, Missouri 65804, USA ABSTRACT.–The distribution range and status of Chitra spp. in Thailand were investigated. Chitra chitra Nutphand, 1986 is so far known only from the Mae Klong and Chao Phraya river systems. Another species, Chitra vandijki McCord and Pritchard, 2002, was reported to occur in the Salween river system located along the Thailand-Myanmar border. At present, the status of Chitra spp. is very rare everywhere and the natural population seems to be declining. Conservation and management action in behalf of this Genus is urgently needed. KEY WORDS: Trionychidae, Chitra chitra, Chitra vandijki, softshell turtle, distribution, conservation Thailand. Information subsequently presented INTRODUCTION by Engstrom et al. (2002), Engstrom and McCord (2002), and McCord and Pritchard The Siamese narrow-headed softshell turtle, (2002) now indicates that populations C. chitra Nutphand, 1986, is probably the representing this species also extend into largest softshell turtle in the world. Pritchard peninsular Malaysia and Indonesia (to Java). Of (2001) estimated the maximum leathery the six major river drainages recognized in carapace length (LCL) of C. chitra as 122 cm. Thailand by Vidthayanon et al.