Cloud in a Bottle (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 5 Measures of Humidity Phases of Water
Chapter 5 Atmospheric Moisture Measures of Humidity 1. Absolute humidity 2. Specific humidity 3. Actual vapor pressure 4. Saturation vapor pressure 5. Relative humidity 6. Dew point Phases of Water Water Vapor n o su ti b ra li o n m p io d a a at ep t v s o io e en s n d it n io o n c freezing Liquid Water Ice melting 1 Coexistence of Water & Vapor • Even below the boiling point, some water molecules leave the liquid (evaporation). • Similarly, some water molecules from the air enter the liquid (condense). • The behavior happens over ice too (sublimation and condensation). Saturation • If we cap the air over the water, then more and more water molecules will enter the air until saturation is reached. • At saturation there is a balance between the number of water molecules leaving the liquid and entering it. • Saturation can occur over ice too. Hydrologic Cycle 2 Air Parcel • Enclose a volume of air in an imaginary thin elastic container, which we will call an air parcel. • It contains oxygen, nitrogen, water vapor, and other molecules in the air. 1. Absolute Humidity Mass of water vapor Absolute humidity = Volume of air The absolute humidity changes with the volume of the parcel, which can change with temperature or pressure. 2. Specific Humidity Mass of water vapor Specific humidity = Total mass of air The specific humidity does not change with parcel volume. 3 Specific Humidity vs. Latitude • The highest specific humidities are observed in the tropics and the lowest values in the polar regions. -

Insar Water Vapor Data Assimilation Into Mesoscale Model
This article has been accepted for inclusion in a future issue of this journal. Content is final as presented, with the exception of pagination. IEEE JOURNAL OF SELECTED TOPICS IN APPLIED EARTH OBSERVATIONS AND REMOTE SENSING 1 InSAR Water Vapor Data Assimilation into Mesoscale Model MM5: Technique and Pilot Study Emanuela Pichelli, Rossella Ferretti, Domenico Cimini, Giulia Panegrossi, Daniele Perissin, Nazzareno Pierdicca, Senior Member, IEEE, Fabio Rocca, and Bjorn Rommen Abstract—In this study, a technique developed to retrieve inte- an extremely important element of the atmosphere because its grated water vapor from interferometric synthetic aperture radar distribution is related to clouds, precipitation formation, and it (InSAR) data is described, and a three-dimensional variational represents a large proportion of the energy budget in the atmo- assimilation experiment of the retrieved precipitable water vapor into the mesoscale weather prediction model MM5 is carried out. sphere. Its representation inside numerical weather prediction The InSAR measurements were available in the framework of the (NWP) models is critical to improve the weather forecast. It is European Space Agency (ESA) project for the “Mitigation of elec- also very challenging because water vapor is involved in pro- tromagnetic transmission errors induced by atmospheric water cesses over a wide range of spatial and temporal scales. An vapor effects” (METAWAVE), whose goal was to analyze and pos- improvement in atmospheric water vapor monitoring that can sibly predict the phase delay induced by atmospheric water vapor on the spaceborne radar signal. The impact of the assimilation on be assimilated in NWP models would improve the forecast the model forecast is investigated in terms of temperature, water accuracy of precipitation and severe weather [1], [3]. -

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Effect of Wind on Near-Shore Breaking Waves
EFFECT OF WIND ON NEAR-SHORE BREAKING WAVES by Faydra Schaffer A Thesis Submitted to the Faculty of The College of Engineering and Computer Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2010 Copyright by Faydra Schaffer 2010 ii ACKNOWLEDGEMENTS The author wishes to thank her mother and family for their love and encouragement to go to college and be able to have the opportunity to work on this project. The author is grateful to her advisor for sponsoring her work on this project and helping her to earn a master’s degree. iv ABSTRACT Author: Faydra Schaffer Title: Effect of wind on near-shore breaking waves Institution: Florida Atlantic University Thesis Advisor: Dr. Manhar Dhanak Degree: Master of Science Year: 2010 The aim of this project is to identify the effect of wind on near-shore breaking waves. A breaking wave was created using a simulated beach slope configuration. Testing was done on two different beach slope configurations. The effect of offshore winds of varying speeds was considered. Waves of various frequencies and heights were considered. A parametric study was carried out. The experiments took place in the Hydrodynamics lab at FAU Boca Raton campus. The experimental data validates the knowledge we currently know about breaking waves. Offshore winds effect is known to increase the breaking height of a plunging wave, while also decreasing the breaking water depth, causing the wave to break further inland. Offshore winds cause spilling waves to react more like plunging waves, therefore increasing the height of the spilling wave while consequently decreasing the breaking water depth. -

NWS Unified Surface Analysis Manual
Unified Surface Analysis Manual Weather Prediction Center Ocean Prediction Center National Hurricane Center Honolulu Forecast Office November 21, 2013 Table of Contents Chapter 1: Surface Analysis – Its History at the Analysis Centers…………….3 Chapter 2: Datasets available for creation of the Unified Analysis………...…..5 Chapter 3: The Unified Surface Analysis and related features.……….……….19 Chapter 4: Creation/Merging of the Unified Surface Analysis………….……..24 Chapter 5: Bibliography………………………………………………….…….30 Appendix A: Unified Graphics Legend showing Ocean Center symbols.….…33 2 Chapter 1: Surface Analysis – Its History at the Analysis Centers 1. INTRODUCTION Since 1942, surface analyses produced by several different offices within the U.S. Weather Bureau (USWB) and the National Oceanic and Atmospheric Administration’s (NOAA’s) National Weather Service (NWS) were generally based on the Norwegian Cyclone Model (Bjerknes 1919) over land, and in recent decades, the Shapiro-Keyser Model over the mid-latitudes of the ocean. The graphic below shows a typical evolution according to both models of cyclone development. Conceptual models of cyclone evolution showing lower-tropospheric (e.g., 850-hPa) geopotential height and fronts (top), and lower-tropospheric potential temperature (bottom). (a) Norwegian cyclone model: (I) incipient frontal cyclone, (II) and (III) narrowing warm sector, (IV) occlusion; (b) Shapiro–Keyser cyclone model: (I) incipient frontal cyclone, (II) frontal fracture, (III) frontal T-bone and bent-back front, (IV) frontal T-bone and warm seclusion. Panel (b) is adapted from Shapiro and Keyser (1990) , their FIG. 10.27 ) to enhance the zonal elongation of the cyclone and fronts and to reflect the continued existence of the frontal T-bone in stage IV. -
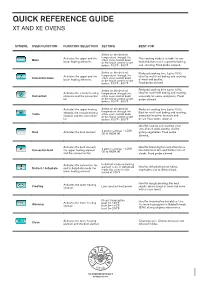
QUICK REFERENCE GUIDE XT and XE Ovens
QUICK REFERENCE GUIDE XT AND XE OVENS SYMBOL OVEN FUNCTION FUNCTION SELECTION SETTING BEST FOR Select on the desired Activates the upper and the temperature through the This cooking mode is suitable for any Bake either oven control knob lower heating elements or the touch control smart kind of dishes and it is great for baking botton. 100°F - 500°F and roasting. Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the upper and the temperature through the Ideal for multi-level baking and roasting Convection bake either oven control knob lower heating elements or the touch control smart of meat and poultry. botton. 100°F - 500°F Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the circular heating temperature through the Ideal for multi-level baking and roasting, Convection elements and the convection either oven control knob especially for cakes and pastry. Food fan or the touch control smart probe allowed. botton. 100°F - 500°F Activates the upper heating Select on the desired Reduced cooking time (up to 10%). temperature through the Best for multi-level baking and roasting, Turbo element, the circular heating either oven control knob element and the convection or the touch control smart especially for pizza, focaccia and fan botton. 100°F - 500°F bread. Food probe allowed. Ideal for searing and roasting small cuts of beef, pork, poultry, and for 4 power settings – LOW Broil Activates the broil element (1) to HIGH (4) grilling vegetables. Food probe allowed. Activates the broil element, Ideal for browning fish and other items 4 power settings – LOW too delicate to turn and thicker cuts of Convection broil the upper heating element (1) to HIGH (4) and the convection fan steaks. -

ESSENTIALS of METEOROLOGY (7Th Ed.) GLOSSARY
ESSENTIALS OF METEOROLOGY (7th ed.) GLOSSARY Chapter 1 Aerosols Tiny suspended solid particles (dust, smoke, etc.) or liquid droplets that enter the atmosphere from either natural or human (anthropogenic) sources, such as the burning of fossil fuels. Sulfur-containing fossil fuels, such as coal, produce sulfate aerosols. Air density The ratio of the mass of a substance to the volume occupied by it. Air density is usually expressed as g/cm3 or kg/m3. Also See Density. Air pressure The pressure exerted by the mass of air above a given point, usually expressed in millibars (mb), inches of (atmospheric mercury (Hg) or in hectopascals (hPa). pressure) Atmosphere The envelope of gases that surround a planet and are held to it by the planet's gravitational attraction. The earth's atmosphere is mainly nitrogen and oxygen. Carbon dioxide (CO2) A colorless, odorless gas whose concentration is about 0.039 percent (390 ppm) in a volume of air near sea level. It is a selective absorber of infrared radiation and, consequently, it is important in the earth's atmospheric greenhouse effect. Solid CO2 is called dry ice. Climate The accumulation of daily and seasonal weather events over a long period of time. Front The transition zone between two distinct air masses. Hurricane A tropical cyclone having winds in excess of 64 knots (74 mi/hr). Ionosphere An electrified region of the upper atmosphere where fairly large concentrations of ions and free electrons exist. Lapse rate The rate at which an atmospheric variable (usually temperature) decreases with height. (See Environmental lapse rate.) Mesosphere The atmospheric layer between the stratosphere and the thermosphere. -

A STUDY of ELEVATED CONVECTION and ITS IMPACTS on SURFACE WEATHER CONDITIONS a Dissertat
A STUDY OF ELEVATED CONVECTION AND ITS IMPACTS ON SURFACE WEATHER CONDITIONS _______________________________________ A Dissertation presented to the Faculty of the Graduate School at the University of Missouri-Columbia _______________________________________________________ In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy _____________________________________________________ by Joshua Kastman Dr. Patrick Market, Dissertation Supervisor December 2017 © copyright by Joshua S. Kastman 2017 All Rights Reserved The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled A Study of Elevated Convection and its Impacts on Surface Weather Conditions presented by Joshua Kastman, a candidate for the degree of doctor of philosophy, Soil, Environmental, and Atmospheric Sciences and hereby certify that, in their opinion, it is worthy of acceptance. ____________________________________________ Professor Patrick Market ____________________________________________ Associate Professor Neil Fox ____________________________________________ Professor Anthony Lupo ____________________________________________ Associate Professor Sonja Wilhelm Stannis ACKNOWLEDGMENTS I would like to begin by thanking Dr. Patrick Market for all of his guidance and encouragement throughout my time at the University of Missouri. His advice has been invaluable during my graduate studies. His mentorship has meant so much to me and I look forward his advice and friendship in the years to come. I would also like to thank Anthony Lupo, Neil Fox and Sonja Wilhelm-Stannis for serving as committee members and for their advice and guidance. I would like to thank the National Science Foundation for funding the project. I would l also like to thank my wife Anna for her, support, patience and understanding while I pursued this degree. Her love and partnerships means everything to me. -

TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # M3/Kg Kj/Kg Kj/Kg Kj/Kg K Sat
720 Tables in SI Units TABLE A-2 Properties of Saturated Water (Liquid–Vapor): Temperature Table Specific Volume Internal Energy Enthalpy Entropy # m3/kg kJ/kg kJ/kg kJ/kg K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Temp. Press. Liquid Vapor Liquid Vapor Liquid Evap. Vapor Liquid Vapor Temp. Њ v ϫ 3 v Њ C bar f 10 g uf ug hf hfg hg sf sg C O 2 .01 0.00611 1.0002 206.136 0.00 2375.3 0.01 2501.3 2501.4 0.0000 9.1562 .01 H 4 0.00813 1.0001 157.232 16.77 2380.9 16.78 2491.9 2508.7 0.0610 9.0514 4 5 0.00872 1.0001 147.120 20.97 2382.3 20.98 2489.6 2510.6 0.0761 9.0257 5 6 0.00935 1.0001 137.734 25.19 2383.6 25.20 2487.2 2512.4 0.0912 9.0003 6 8 0.01072 1.0002 120.917 33.59 2386.4 33.60 2482.5 2516.1 0.1212 8.9501 8 10 0.01228 1.0004 106.379 42.00 2389.2 42.01 2477.7 2519.8 0.1510 8.9008 10 11 0.01312 1.0004 99.857 46.20 2390.5 46.20 2475.4 2521.6 0.1658 8.8765 11 12 0.01402 1.0005 93.784 50.41 2391.9 50.41 2473.0 2523.4 0.1806 8.8524 12 13 0.01497 1.0007 88.124 54.60 2393.3 54.60 2470.7 2525.3 0.1953 8.8285 13 14 0.01598 1.0008 82.848 58.79 2394.7 58.80 2468.3 2527.1 0.2099 8.8048 14 15 0.01705 1.0009 77.926 62.99 2396.1 62.99 2465.9 2528.9 0.2245 8.7814 15 16 0.01818 1.0011 73.333 67.18 2397.4 67.19 2463.6 2530.8 0.2390 8.7582 16 17 0.01938 1.0012 69.044 71.38 2398.8 71.38 2461.2 2532.6 0.2535 8.7351 17 18 0.02064 1.0014 65.038 75.57 2400.2 75.58 2458.8 2534.4 0.2679 8.7123 18 19 0.02198 1.0016 61.293 79.76 2401.6 79.77 2456.5 2536.2 0.2823 8.6897 19 20 0.02339 1.0018 57.791 83.95 2402.9 83.96 2454.1 2538.1 0.2966 8.6672 20 21 0.02487 1.0020 -

Oven Settings Lighting the Burners Plates Which Are Designed to Catch Drippings and All Burners Are Ignited by Electric Circulate a Smoke Flavor Back Into the Food
Surface Operation Range Controls Oven Settings Lighting the Burners plates which are designed to catch drippings and All burners are ignited by electric circulate a smoke flavor back into the food. Beneath the Interior Oven Left Front Burner Left Oven Left Oven Griddle Self-Clean Right Oven Right Front Burner BAKE (Two- require gentle cooking such as pastries, souffles, yeast MED BROIL ignition. There are no open-flame, flavor generator plates is a two piece drip pan which Light Switch Control Knob Function Temperature Indicator Light Indicator Temperature Control Knob Element Bake) breads, quick breads and cakes. Breads, cookies, and other Inner and outer broil “standing” pilots. catches any drippings that might pass beyond the flavor Full power heat is baked goods come out evenly textured with golden crusts. elements pulse on (15,000 BTU) Selector Knob Control Knob Light Indicator Light (15,000 BTU) generator plates. This unique grilling system is designed radiated from the No special bakeware is required. Use this function for single and off to produce VariSimmer™ to provide outdoor quality grilling indoors. bake element in the rack baking, multiple rack baking, roasting, and preparation less heat for slow Simmering is a cooking technique in CLEAN OVEN GRIDDLE OVEN CLEAN bottom of the oven of complete meals. This setting is also recommended when broiling. Allow about which foods are cooked in hot liquids kept at or just Dual Fuel cavity and baking large quantities of baked goods at one time. 4 inches (10 cm) Oven Functions Convection-Self Clean barely below the boiling point of water. -

FORECASTERS' FORUM Elevated Convection And
1280 WEATHER AND FORECASTING VOLUME 23 FORECASTERS’ FORUM Elevated Convection and Castellanus: Ambiguities, Significance, and Questions STEPHEN F. CORFIDI NOAA/NWS/NCEP/Storm Prediction Center, Norman, Oklahoma SARAH J. CORFIDI NOAA/NWS/NCEP/Storm Prediction Center, and Cooperative Institute for Mesoscale Meteorological Studies, University of Oklahoma, Norman, Oklahoma DAVID M. SCHULTZ* Cooperative Institute for Mesoscale Meteorological Studies, University of Oklahoma, and NOAA/National Severe Storms Laboratory, Norman, Oklahoma (Manuscript received 23 January 2008, in final form 26 April 2008) ABSTRACT The term elevated convection is used to describe convection where the constituent air parcels originate from a layer above the planetary boundary layer. Because elevated convection can produce severe hail, damaging surface wind, and excessive rainfall in places well removed from strong surface-based instability, situations with elevated storms can be challenging for forecasters. Furthermore, determining the source of air parcels in a given convective cloud using a proximity sounding to ascertain whether the cloud is elevated or surface based would appear to be trivial. In practice, however, this is often not the case. Compounding the challenges in understanding elevated convection is that some meteorologists refer to a cloud formation known as castellanus synonymously as a form of elevated convection. Two different definitions of castel- lanus exist in the literature—one is morphologically based (cloud formations that develop turreted or cumuliform shapes on their upper surfaces) and the other is physically based (inferring the turrets result from the release of conditional instability). The terms elevated convection and castellanus are not synony- mous, because castellanus can arise from surface-based convection and elevated convection exists that does not feature castellanus cloud formations. -

Humidity, Condensation, and Clouds-I
Humidity, Condensation, and Clouds-I GEOL 1350: Introduction To Meteorology 1 Overview • Water Circulation in the Atmosphere • Properties of Water • Measures of Water Vapor in the Atmosphere (Vapor Pressure, Absolute Humidity, Specific Humidity, Mixing Ratio, Relative Humidity, Dew Point) 2 Where does the moisture in the atmosphere come from ? Major Source Major sink Evaporation from ocean Precipitation 3 Earth’s Water Distribution 4 Fresh vs. salt water • Most of the earth’s water is found in the oceans • Only 3% is fresh water and 3/4 of that is ice • The atmosphere contains only ~ 1 week supply of precipitation! 5 Properties of Water • Physical States only substance that are present naturally in three states • Density liquid : ~ 1.0 g / cm3 , solid: ~ 0.9 g / cm3 , vapor: ~10-5 g / cm3 6 Properties of Water (cont’) • Radiative Properties – transparent to visible wavelengths – virtually opaque to many infrared wavelengths – large range of albedo possible • water 10 % (daily average) • Ice 30 to 40% • Snow 20 to 95% • Cloud 30 to 90% 7 Three phases of water • Evaporation liquid to vapor • Condensation vapor to liquid • Sublimation solid to vapor • Deposition vapor to solid • Melting solid to liquid • Freezing liquid to solid 8 Heat exchange with environment during phase change As water moves toward vapor it absorbs latent heat to keep the molecules in rapid mo9ti on Energy associated with phase change Sublimation Deposition 10 Sublimation – evaporate ice directly to water vapor Take one gram of ice at zero degrees centigrade Energy required