The Biology and Systematics of Ebenaceae: a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi
YIKA-VWAZA TRUST RESEARCH STUDY REPORT N (2017/18) Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi By Sopani Sichinga ([email protected]) September , 2019 ABSTRACT In 2018 – 19, a survey on vascular plants was conducted in Vwaza Marsh Wildlife Reserve. The reserve is located in the north-western Malawi, covering an area of about 986 km2. Based on this survey, a total of 461 species from 76 families were recorded (i.e. 454 Angiosperms and 7 Pteridophyta). Of the total species recorded, 19 are exotics (of which 4 are reported to be invasive) while 1 species is considered threatened. The most dominant families were Fabaceae (80 species representing 17. 4%), Poaceae (53 species representing 11.5%), Rubiaceae (27 species representing 5.9 %), and Euphorbiaceae (24 species representing 5.2%). The annotated checklist includes scientific names, habit, habitat types and IUCN Red List status and is presented in section 5. i ACKNOLEDGEMENTS First and foremost, let me thank the Nyika–Vwaza Trust (UK) for funding this work. Without their financial support, this work would have not been materialized. The Department of National Parks and Wildlife (DNPW) Malawi through its Regional Office (N) is also thanked for the logistical support and accommodation throughout the entire study. Special thanks are due to my supervisor - Mr. George Zwide Nxumayo for his invaluable guidance. Mr. Thom McShane should also be thanked in a special way for sharing me some information, and sending me some documents about Vwaza which have contributed a lot to the success of this work. I extend my sincere thanks to the Vwaza Research Unit team for their assistance, especially during the field work. -

Tamarind 1990 - 2004
Tamarind 1990 - 2004 Author A. K. A. Dandjouma, C. Tchiegang, C. Kapseu and R. Ndjouenkeu Title Ricinodendron heudelotii (Bail.) Pierre ex Pax seeds treatments influence on the q Year 2004 Source title Rivista Italiana delle Sostanze Grasse Reference 81(5): 299-303 Abstract The effects of heating Ricinodendron heudelotii seeds on the quality of the oil extracted was studied. The seeds were preheated by dry and wet methods at three temperatures (50, 70 and 90 degrees C) for 10, 20, 30 and 60 minutes. The oil was extracted using the Soxhlet method with hexane. The results showed a significant change in oil acid value when heated at 90 degrees C for 60 minutes, with values of 2.76+or-0.18 for the dry method and 2.90+or-0.14 for the wet method. Heating at the same conditions yielded peroxide values of 10.70+or-0.03 for the dry method and 11.95+or-0.08 for the wet method. Author A. L. Khandare, U. Kumar P, R. G. Shanker, K. Venkaiah and N. Lakshmaiah Title Additional beneficial effect of tamarind ingestion over defluoridated water supply Year 2004 Source title Nutrition Reference 20(5): 433-436 Abstract Objective: We evaluated the effect of tamarind (Tamarindus indicus) on ingestion and whether it provides additional beneficial effects on mobilization of fluoride from the bone after children are provided defluoridated water. Methods: A randomized, diet control study was conducted in 30 subjects from a fluoride endemic area after significantly decreasing urinary fluoride excretion by supplying defluoridated water for 2 wk. -

Caterpillars Moths Butterflies Woodies
NATIVE Caterpillars Moths and utter flies Band host NATIVE Hackberry Emperor oodies PHOTO : Megan McCarty W Double-toothed Prominent Honey locust Moth caterpillar Hackberry Emperor larva PHOTO : Douglas Tallamy Big Poplar Sphinx Number of species of Caterpillars n a study published in 2009, Dr. Oaks (Quercus) 557 Beeches (Fagus) 127 Honey-locusts (Gleditsia) 46 Magnolias (Magnolia) 21 Double-toothed Prominent ( Nerice IDouglas W. Tallamy, Ph.D, chair of the Cherries (Prunus) 456 Serviceberry (Amelanchier) 124 New Jersey Tea (Ceanothus) 45 Buttonbush (Cephalanthus) 19 bidentata ) larvae feed exclusively on elms Department of Entomology and Wildlife Willows (Salix) 455 Larches or Tamaracks (Larix) 121 Sycamores (Platanus) 45 Redbuds (Cercis) 19 (Ulmus), and can be found June through Ecology at the University of Delaware Birches (Betula) 411 Dogwoods (Cornus) 118 Huckleberry (Gaylussacia) 44 Green-briar (Smilax) 19 October. Their body shape mimics the specifically addressed the usefulness of Poplars (Populus) 367 Firs (Abies) 117 Hackberry (Celtis) 43 Wisterias (Wisteria) 19 toothed shape of American elm, making native woodies as host plants for our Crabapples (Malus) 308 Bayberries (Myrica) 108 Junipers (Juniperus) 42 Redbay (native) (Persea) 18 them hard to spot. The adult moth is native caterpillars (and obviously Maples (Acer) 297 Viburnums (Viburnum) 104 Elders (Sambucus) 42 Bearberry (Arctostaphylos) 17 small with a wingspan of 3-4 cm. therefore moths and butterflies). Blueberries (Vaccinium) 294 Currants (Ribes) 99 Ninebark (Physocarpus) 41 Bald cypresses (Taxodium) 16 We present here a partial list, and the Alders (Alnus) 255 Hop Hornbeam (Ostrya) 94 Lilacs (Syringa) 40 Leatherleaf (Chamaedaphne) 15 Honey locust caterpillar feeds on honey number of Lepidopteran species that rely Hickories (Carya) 235 Hemlocks (Tsuga) 92 Hollies (Ilex) 39 Poison Ivy (Toxicodendron) 15 locust, and Kentucky coffee trees. -

Brief Review on the Genus Diospyros: a Rich Source of Naphthoquinones
Asian J. Adv. Basic Sci.: 2017, 5(2), 34-53 ISSN (Print): 2454 – 7492 ISSN (Online): 2347 – 4114 www.ajabs.org Brief Review on the Genus Diospyros: A Rich Source of Naphthoquinones Venu Sharma School of Biotechnology, University of Jammu, Jammu & Kashmir, INDIA * Correspondance: E-mail: [email protected] (Received 01 Sept, 2017; Accepted 14 Sept, 2017; Published 21 Sept, 2017) ABSTRACT: Diospyros genus (Ebenaceae) comprises about 500 species. A number of them are used for their multiple pharmacological activities. Ethano-phramacologically various plant parts are formulated and prescribed in the form of extracts and the decoctions for remedy of different diseases in many tribes. These activities are due to presence of bioactive secondary metabolites in the plant. Systematic and detailed investigation on the composition and pharmacological significance of medicinal plants should be conducted to standardize the formulations, based on ingredients. In the present review, naphthoquinone, naphthalene and naphthol derivatives isolated from Diospyros species are documented here with the pharmacologically important species of the genus. Keywords: Diospyros genus; species; Ebenaceae; pharmacological activities; naphthoquinones. INTRODUCTION: Diospyros genus belongs to the retic, laxative and stypic. Its dried flowers are reported to family Ebenaceae. The plants of Ebenaceae are wide be used in urinary, skin and blood diseases. A dilute ex- spread in tropics and sub tropics, occasionally into tem- tract is used as an astringent lotion for eyes5. Heartwood perate areas. According to Hegnaeur1 the family consists of D.mollis has been found to be active against hook- of seven genera, namely Diospyros, Euclea, Maba, worms but less active against tapeworms14. Oncothea, Rhaphidanthe, Royena and Tetraclis. -

Utilization of Lignocellulosic Waste from Bidi Industry for Removal of Dye from Aqueous Solution
Int. J. Environ. Res., 2(4): 385-390, Autumn 2008 ISSN: 1735-6865 Utilization of Lignocellulosic Waste from Bidi Industry for Removal of Dye from Aqueous Solution Nagda, G. K.* and Ghole, V. S. Department of Environmental Sciences, University of Pune, Pune, India Received 26 Oct 2007; Revised 15 March 2008; Accepted 15 April 2008 ABSTRACT: A new, local agro-industrial waste was valorized by chemical treatment and tested for its ability to remove cationic dye from aqueous solution. Tendu (Diospyros melanoxylon) leaves refuse, a solid waste from bidi industry which caused disposal problem, was studied as a biosorbent. Raw tendu waste (TLR), along with sulfuric acid carbonized tendu waste (TLR-CM) and tendu waste treated with dilute sulfuric acid (TLR-2N) were utilized as sorbent for uptake of crystal violet from aqueous solutions. Adsorption studies were carried out at various dye concentrations and contact times. It followed the pseudo-second-order kinetics and followed the Langmuir adsorption isotherm. Interestingly, milder acid treatment of the tendu waste enhanced biosorption, whereas drastic acid carbonization of tendu waste resulted in reduced adsorption of dye. The maximum adsorption capacities for crystal violet for TLR-2N, TLR and TLR-CM are 67.57, 42.92 and 22.47 mg/g respectively. Commercial activated carbon had maximum adsorption capacity for crystal violet of 151.52 mg/g. Thus a renewable solid waste with mild acid treatment can offer a cost effective alternative to activated carbon. Key words: Biosorption, Diospyros melanoxylon, Solid tendu waste, Dye, Crystal violet INTRODUCTION Colored wastewater is a consequence of synthetic textile dyes because of the chemical industrial processes both in the dye manufacturing stability of these pollutants (Anjaneyulu et al., industries and in the dye-consuming industries. -

Differences in the Phenolic Profile by UPLC Coupled to High Resolution
antioxidants Article Differences in the Phenolic Profile by UPLC Coupled to High Resolution Mass Spectrometry and Antioxidant Capacity of Two Diospyros kaki Varieties Adelaida Esteban-Muñoz 1,2,*, Silvia Sánchez-Hernández 1,2, Cristina Samaniego-Sánchez 1,3 , Rafael Giménez-Martínez 1,3 and Manuel Olalla-Herrera 1,3 1 Departamento de Nutrición y Bromatología, Universidad de Granada, 18071 Granada, Spain; [email protected] (S.S.-H.); [email protected] (C.S.-S.); [email protected] (R.G.-M.); [email protected] (M.O.-H.) 2 Programme in Nutrition and Food Science, University of Granada, 18071 Granada, Spain 3 Biosanitary Research Institute, IBS, 18071 Granada, Spain * Correspondence: [email protected]; Tel.: +34-958-243-863 Abstract: Background: phenolic compounds are bioactive chemical species derived from fruits and vegetables, with a plethora of healthy properties. In recent years, there has been a growing inter- est in persimmon (Diospyros kaki L.f.) due to the presence of many different classes of phenolic compounds. However, the analysis of individual phenolic compounds is difficult due to matrix interferences. Methods: the aim of this research was the evaluation of individual phenolic compounds and antioxidant capacity of the pulp of two varieties of persimmon (Rojo Brillante and Triumph) by an improved extraction procedure together with a UPLC-Q-TOF-MS platform. Results: the phenolic compounds composition of persimmon was characterized by the presence of hydroxybenzoic and hy- droxycinnamic acids, hydroxybenzaldehydes, dihydrochalcones, tyrosols, flavanols, flavanones, and Citation: Esteban-Muñoz, A.; flavonols. A total of 31 compounds were identified and 17 compounds were quantified. Gallic acid was Sánchez-Hernández, S.; Rojo Brillante Samaniego-Sánchez, C.; the predominant phenolic compounds found in the variety (0.953 mg/100 g) whereas the Giménez-Martínez, R.; concentration of p-hydroxybenzoic acid was higher in the Triumph option (0.119 mg/100 g). -

ORNAMENTAL GARDEN PLANTS of the GUIANAS: an Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana
f ORNAMENTAL GARDEN PLANTS OF THE GUIANAS: An Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana Vf•-L - - •• -> 3H. .. h’ - — - ' - - V ' " " - 1« 7-. .. -JZ = IS^ X : TST~ .isf *“**2-rt * * , ' . / * 1 f f r m f l r l. Robert A. DeFilipps D e p a r t m e n t o f B o t a n y Smithsonian Institution, Washington, D.C. \ 1 9 9 2 ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Table of Contents I. Map of the Guianas II. Introduction 1 III. Basic Bibliography 14 IV. Acknowledgements 17 V. Maps of Guyana, Surinam and French Guiana VI. Ornamental Garden Plants of the Guianas Gymnosperms 19 Dicotyledons 24 Monocotyledons 205 VII. Title Page, Maps and Plates Credits 319 VIII. Illustration Credits 321 IX. Common Names Index 345 X. Scientific Names Index 353 XI. Endpiece ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Introduction I. Historical Setting of the Guianan Plant Heritage The Guianas are embedded high in the green shoulder of northern South America, an area once known as the "Wild Coast". They are the only non-Latin American countries in South America, and are situated just north of the Equator in a configuration with the Amazon River of Brazil to the south and the Orinoco River of Venezuela to the west. The three Guianas comprise, from west to east, the countries of Guyana (area: 83,000 square miles; capital: Georgetown), Surinam (area: 63, 037 square miles; capital: Paramaribo) and French Guiana (area: 34, 740 square miles; capital: Cayenne). Perhaps the earliest physical contact between Europeans and the present-day Guianas occurred in 1500 when the Spanish navigator Vincente Yanez Pinzon, after discovering the Amazon River, sailed northwest and entered the Oyapock River, which is now the eastern boundary of French Guiana. -

Pp. 72-75, 2020 Download
T REPRO N DU The International Journal of Plant Reproductive Biology 12(1) Jan., 2020, pp.72-75 LA C P T I F V O E B Y T I DOI 10.14787/ijprb.2020 12.1. O E I L O C G O S I S T E S H Floral anatomy and flower visitors of three persimmon (Diospyros kaki L.) T varieties cultivated in Central Europe Virág Andor and Ágnes Farkas* Department of Pharmacognosy, Faculty of Pharmacy, University of Pécs, H-7624 Pécs, Rókus str. 2., Hungary *e-mail : [email protected] Received : 20.12.2019; Accepted and Published online: 31.12.2019 ABSTRACT We report flower and pollination biological traits of three persimmon (Diospyros kaki L.) varieties cultivated under suboptimal conditions in the temperate climate of Central Europe. In order to observe flower visiting insects and floral morphology, and to determine the nectar producing capacity of persimmon flowers, field studies were conducted in 2018 and 2019. The anatomical studies were performed with light microscopy. Quantitative floral traits were analysed with two-sample t-test. The main flower visitors were honeybees (Apis mellifera L.) and bumblebees (Bombus sp.), which can act as pollinators, while searching for nectar. The studied persimmon varieties belong to the gynoecious type, the solitary pistillate flowers consisting of four- membered calyx and corolla, reduced androecium and a pistil with superior ovary and 3 to 5 stigmata. The size of the calyx was significantly different in different varieties, but corolla diameter did not differ within the same year of study. The diameter of both the calyx and corolla of the same variety was bigger in 2019 compared to 2018, due to favourable climatic conditions. -

Noctuoidea: Erebidae: Others
Staude et al. / Metamorphosis 27: S165–S188 S165 ____________________________________________________________________________________________________________________________ Noctuoidea: Erebidae: Others Reference/ Lepidoptera Host plant Locality rearing no. Taxon Subfamily Family Taxon Family M1148 Anoba angulilinea Anobinae Erebidae Dalbergia Fabaceae Tshukudu Game melanoxylon Reserve, Hoedspruit M998 Anoba atripuncta Anobinae Erebidae Ormocarpum Fabaceae Tshukudu Game trichocarpum Reserve, Hoedspruit Gv71 Baniana arvorum Anobinae Erebidae Elephantorrhiza Fabaceae Steenkoppies, farm, elephantina Magaliesburg 14HSS52 Baniana arvorum Anobinae Erebidae Elephantorrhiza Fabaceae Steenkoppies, farm, elephantina Magaliesburg 13HSS84 Plecoptera arctinotata Anobinae Erebidae Senegalia caffra Fabaceae Steenkoppies, farm, Magaliesburg M1020a Plecoptera flaviceps Anobinae Erebidae Dalbergia Fabaceae Casketts, farm, melanoxylon Hoedspruit M317 Bareia incidens Calpinae Erebidae Ficus lutea Moraceae Casketts, farm, (unplaced as to Hoedspruit tribe) 14HSS87 Egnasia vicaria Calpinae Erebidae Afrocanthium Rubiaceae Dlinsa Forest, (unplaced as to mundianum Eshowe tribe) 12HSS163 Exophyla multistriata Calpinae Erebidae Celtis africana Cannabaceae Golden Valley, (unplaced as to Magaliesburg tribe) M416 Exophyla multistriata Calpinae Erebidae Trema orientalis Cannabaceae Sekororo, Tzaneen (unplaced as to (Fed on Celtis tribe) africana) M743 Lacera alope Calpinae Erebidae Pterolobium Fabaceae Moholoholo Rehab (unplaced as to stellatum Centre, Hoedspruit tribe) -
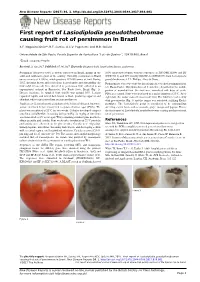
First Report of Lasiodiplodia Pseudotheobromae Causing Fruit Rot of Persimmon in Brazil
New Disease Reports (2017) 36, 1. http://dx.doi.org/10.5197/j.2044-0588.2017.036.001 First report of Lasiodiplodia pseudotheobromae causing fruit rot of persimmon in Brazil A.F. Nogueira Júnior*, R.F. Santos, A.C.V. Pagenotto and M.B. Spósito Universidade de São Paulo, Escola Superior de Agricultura “Luiz de Queiroz”, 13418-900, Brazil *E-mail: [email protected] Received: 23 Jun 2017. Published: 07 Jul 2017. Keywords: Diospyros kaki, fungal plant disease, postharvest Persimmon (Diospyros kaki) is widely cultivated in Brazil, mainly in the 100% nucleotide identity with the sequences of ITS (NR111264) and BT south and southeast regions of the country. Currently, persimmon in Brazil (EU673111), and 99% identity with EF-1α (EF622057) from Lasiodiplodia covers an area of 8,300 ha, which produces 182,000 tonnes of fruit. During pseudotheobromae A.J.L. Phillips, Alves & Crous. 2015, irregular brown and soft lesions located under and surrounding the Pathogenicity tests were done by inoculating six detached persimmon fruit fruit calyx (stem-end) were observed in persimmon fruit collected in an (cv. Rama Forte). Mycelium discs of 8 mm were deposited in the middle experimental orchard in Piracicaba, Sao Paulo State, Brazil (Fig. 1). portion of wounded fruit. Six fruit were inoculated with discs of sterile Disease incidence in sampled fruit (n=50) was around 10%. Lesions PDA as a control. Fruit were incubated in a moist chamber at 25°C. After expanded rapidly and turned dark brown to black producing apparent and eight days, the isolate caused lesions in all fruit. The fruit were covered by abundant white to grey mycelium on fruit postharvest. -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

(Ebenaceae) by Evaluating Short Sequence Region of Plastid Rbcl Gene
POJ 7(2):102-107 (2014) ISSN:1836-3644 Nucleotide based validation of the endangered plant Diospyros mespiliformis (Ebenaceae) by evaluating short sequence region of plastid rbcL gene Abdullah Alaklabi1, Ibrahim A. Arif 2,3, Sameera O. Bafeel4, Ahmad H. Alfarhan2,3, Anis Ahamed2,3, Jacob Thomas2 and Mohammad A. Bakir2,3* 1Department of Biology, College of Arts and Science, Al-Baha University (BU), Baljurashi, Saudi Arabia 2Department of Botany and Microbiology, College of Science, King Saud University (KSU), Riyadh, Saudi Arabia 3Saudi Biological Society and Prince Sultan Research Chair for Environment and Wildlife, King Saud University 4Department of Biology, King Abdulaziz University (KAU), Jeddah, Saudi Arabia *Corresponding author. Email: [email protected] Abstract Diospyros mespiliformis (Hochst. ex A.DC.; Ebenaceae) is a large deciduous medicinal plant. This plant species is currently listed as endangered in Saudi Arabia. Molecular identification of this plant species based on short sequence regions (571 and 664 bp) of plastid rbcL (ribulose-1, 5-biphosphate carboxylase) gene was investigated in this study. The endangered plant specimens were collected from Al-Baha, Saudi Arabia (GPS coordinate: 19.8543987, 41.3059349). Phylogenetic tree inferred from the rbcL gene sequences showed that this species is very closely related with D. brandisiana. Close relationship was also observed among D. bejaudii, D. Philippinensis and D. releyi (≥99.7% sequence homology). The partial rbcL gene sequence region (571 bp) that was amplified by rbcL primer-pair rbcLaF-rbcLaR failed to discriminate D. mespiliformis from the closely related plant species, D. brandisiana. In contrast, primer-pair rbcL1F-rbcL724R yielded longer amplicon, discriminated the species from D.