Ecology of Gelatinous Plankton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Place-Names of Inverness and Surrounding Area Ainmean-Àite Ann an Sgìre Prìomh Bhaile Na Gàidhealtachd
Place-Names of Inverness and Surrounding Area Ainmean-àite ann an sgìre prìomh bhaile na Gàidhealtachd Roddy Maclean Place-Names of Inverness and Surrounding Area Ainmean-àite ann an sgìre prìomh bhaile na Gàidhealtachd Roddy Maclean Author: Roddy Maclean Photography: all images ©Roddy Maclean except cover photo ©Lorne Gill/NatureScot; p3 & p4 ©Somhairle MacDonald; p21 ©Calum Maclean. Maps: all maps reproduced with the permission of the National Library of Scotland https://maps.nls.uk/ except back cover and inside back cover © Ashworth Maps and Interpretation Ltd 2021. Contains Ordnance Survey data © Crown copyright and database right 2021. Design and Layout: Big Apple Graphics Ltd. Print: J Thomson Colour Printers Ltd. © Roddy Maclean 2021. All rights reserved Gu Aonghas Seumas Moireasdan, le gràdh is gean The place-names highlighted in this book can be viewed on an interactive online map - https://tinyurl.com/ybp6fjco Many thanks to Audrey and Tom Daines for creating it. This book is free but we encourage you to give a donation to the conservation charity Trees for Life towards the development of Gaelic interpretation at their new Dundreggan Rewilding Centre. Please visit the JustGiving page: www.justgiving.com/trees-for-life ISBN 978-1-78391-957-4 Published by NatureScot www.nature.scot Tel: 01738 444177 Cover photograph: The mouth of the River Ness – which [email protected] gives the city its name – as seen from the air. Beyond are www.nature.scot Muirtown Basin, Craig Phadrig and the lands of the Aird. Central Inverness from the air, looking towards the Beauly Firth. Above the Ness Islands, looking south down the Great Glen. -

PTH Winter 2019
WINTER 20 19 The Pine Tree Highlander A P ublication of the St. Andrews Society of Maine Ulster Scots Claim Long-Time Residency by Dr. Mary Drymon DeRose authentic Scottish music. Nick DeRose, Josh The 1718 Institute, South Portland, Maine Drymon, and Adrian Dowling, former Chairperson of the South Portland Arts and Historical In the Autumn of 1718, two ships of migrants Preservation committee also attended. arrived in Casco Bay, marking the beginning of the first large migration from Ireland to North Dr. DeRose read a list of the names of those who America. The HMS McCallum and the HMS overwintered in Maine and Ms. Reckitt read the fol - Robert brought settlers from Ulster in Northern lowing: Ireland. These ships carried the ancestors of many modern residents of Maine. In recognition of the 300th anniversary of this arrival, Lois Galgary Reckitt, Maine State “JOINT RESOLUTION RECOGNIZING THE CON - Representative for District 31 (herself of Scottish TRIBUTIONS TO THE STATE OF THE SCOTCH- decent) presented a Joint Resolution recognizing IRISH MIGRANTS OF 1718 ONC oTnHtiEn uOeCdC AoSnI OPNag Oe F 2 the Scotch-Irish Migrants of 1718 to Dr. Mary Drymon-DeRose of The 1718 Project at the site on the Fore River in South Portland where the HMS Robert had docked 300 years ago. The ship became frozen in the ice during the bit - terly cold winter of 1718-1719, with many families remaining aboard until the spring. The presentation ceremony was attended by descendants of some of those settlers and other interested participants of Scots and Irish descent. David McCausland, wearing his familial Buchanan Tartan kilt and Nancy Tudor represented their ancestor, Robert pas - senger James McCausland. -

Inventory Acc.11713 Clan Gregor Centre Archive
Acc.11713 June 2011 Inventory Acc.11713 Clan Gregor Centre Archive (History and Genealogy) National Library of Scotland Manuscripts Division George IV Bridge Edinburgh EH1 1EW Tel: 0131-466 2812 Fax: 0131-466 2811 E-mail: [email protected] © Trustees of the National Library of Scotland Donated to the National Library of Scotland on behalf of the Clan Gregor Centre in February 1999. This is the second of three (as of April 2004) Clan Gregor deposits: for the first, see Acc.10664; for the third seeAcc.12335. This inventory has been compiled by Sheila McGregor, FSA Scot, for the Clan Gregor Centre. Introduction: This material represents the contribution of a rather small number of active individuals to the Clan Gregor Centre over many years of collecting and compiling. It represent both traditional knowledge and many years of research. It has occasionally been possible to attribute a file to a named individual but in most cases the folders are composite collections from many sources and these are attributed to me in my capacity as curator and general editor. The members of the Clan as a whole, who are extremely numerous, owe the active few a great deal since they have been among the very few to keep hold of their real past and to avoid the romantic escapism that pervades modern Highland culture, if that is the right word. It has been possible, with the support of these few active members and the willing collaboration of the National Library of Scotland, not only to preserve their knowledge but often to extend it since modern research tools have made it sometimes possible to find solutions to long-standing problems. -

The Dark Ecology of Naked Lunch
humanities Article The Dark Ecology of Naked Lunch George Hart Department of English, California State University Long Beach, Long Beach, CA 90840, USA; [email protected] Received: 15 September 2020; Accepted: 22 October 2020; Published: 30 October 2020 Abstract: In this article, I argue that William S. Burroughs’ novel Naked Lunch engages in a “perverse aesthetics” that is analogous to Timothy Morton’s theory of dark ecology. The novel’s main themes of consumption and control are directly related to the Anthropocene’s twin disasters of global warming and mass extinction, and the trope for addiction, junk, reveals Burroughs’ deep analysis of the political and social forces that attempt to control life, what Burroughs calls biocontrol. By placing the novel’s obsession with hanging/lynching in the context of dark ecology, its critique of racism can also be seen as a critique of speciesism. Keywords: William S. Burroughs; Naked Lunch; dark ecology; consumption; control; Timothy Morton; speciesism; consumerism; mass extinction In the introduction of The Green Ghost: William Burroughs and the Ecological Mind, Chad Weidner rejects Timothy Morton’s dark ecology as a model for reading Naked Lunch. Drawing from The Ecological Thought, Weidner dismisses Morton’s claims that thinking ecologically is viral (an idea that would seem to appeal to the author of Naked Lunch), and that ecology includes such things as human emotions, mental illness, and capitalism, because such a theory “cast[s] a very wide net indeed”. According to Weidner, Morton “seems to accept the notion of nature as a cultural construct ::: but at the same time advises that we abandon romantic notions of nature altogether, to develop a more skeptical postmodern position. -

Scottish Genealogist Cumulative Index 1953 - 2005
SCOTTISH GENEALOGIST CUMULATIVE INDEX 1953 - 2005 Compiled by Dr. James D. Floyd John & Margaret Kinnaird D. Richard Torrance and Other unidentified members of the Society Copyright The Scottish Genealogy Society 2007 The Scottish Genealogy Society Library & Family History Centre 15 Victoria Terrace Edinburgh EH1 2JL Tel: 0131 220 3677 http://www.scotsgenealogy.com SCOTTISH GENEALOGIST Index Volumes 1-52 1953-2005 INTRODUCTION Over the existence of the Scottish Genealogy Society indexes to the Scottish Genealogist have been published at regular intervals and distributed to those who were members of the Society at the time of publication. The index to the first 28 volumes was one large index with no sub-divisions. As a great number of queries were published it was decided to include these in a section of their own from volume 29 onwards. From volume 41 the index was split into the following sections: General index; Article Titles; Contributors; Reviews; Work in Progress; Queries. A separate section for Illustrations was included in the index for volumes 45-52. NUMBERING There has not been uniformity in the numbering format used by the different compilers of the indexes. A volume covers one year during which 4 journals were issued usually in March, June, September and December. In the current index these have been harmonised to follow the most commonly used pattern: Volume number - Roman numerals capitals Journal number - Roman numerals lower case Page number - Arabic numerals Example: XXXIX.iv.116 -Volume 39, December issue, page 116. Page numbering Page numbering in the journals has not been consistent over the years. -

Mapping Cultural Hallmarks Through Names, Surnames and Orthodoxy
Journal of Ethnic and Cultural Studies Copyright 2017 2017, Vol. 4, No. 2, 53-64 ISSN: 2149-1291 Gagauzian onomastics: Mapping cultural hallmarks through names, surnames and Orthodoxy MitranIlie Iulian1 Doctoral School of Sociology, University of Bucharest Gagauzian onomastics presents us an intrequit structure which is characterized by various lingusitic layers that overlap, or at times, even blend in with each other. Unlike other Turcik groups, the Gagauzians pride themselves with their strong commitment to the Orthodox Church. Lexical layering is a defining characteristic of Gagauzian onomastics.As a result, the names and surnames that are found among these people are were, to a certain exctent, transfered from the those groups that they heavly interacted with until the present. The layered layout of Gagauzian onomastics refects the different stage of the coming into being of this peoples, taking this in to consideration, it is important to note that certain surnames are of older date than others, this being the case of those that are of Greek origin. Nowadays, in Moldova, the state with the largest Gagauzian communities, first names are of Russian origin, and are directliany linked to strong russofilia that is present within Gagauz communities beginning with the second falf of the last century.The data that was used for this paper was collected from various soruces – scientific papers, journals, annals etc. Within this paper we are attempting to highlight the conservative character of Gagauzian name-giving practices and the way in which this corelates to the virtues that are central to these peoples. Keywords; Mapping cultural hallmarks, Gagauzian onomastics, Orthodoxy, and Turcik groups From Cavarna to the desolate plains of Budjak: Key-events that shaped Gagauzian history and culture Just a few years ago, Congaz, a settlement in southern Moldova, was roomered to be benefinitng from a series of privileges, which were made possible through the good will of some high-ranking politicians from Kishinev. -

The Enlightenment of Jean-Baptiste-Louis Gresset, Léger-Marie Deschamps, and Simon-Nicolas-Henri Linguet
‘TOUCHSTONES OF TRUTH’: THE ENLIGHTENMENT OF JEAN-BAPTISTE-LOUIS GRESSET, LÉGER-MARIE DESCHAMPS, AND SIMON-NICOLAS-HENRI LINGUET DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Mircea Alexandru Platon, M.A. Graduate Program in History The Ohio State University 2012 Dissertation Committee: Dr. Dale K. Van Kley, Adviser Dr. Alice Conklin, Adviser Dr. Nicholas Breyfogle Copyright Mircea Alexandru Platon 2012 ABSTRACT My dissertation, “ ‘Touchstones of Truth’: The Enlightenment of J.-B.-L. Gresset, L.-M. Deschamps, and S.-N.-H. Linguet,” focuses on three key but little studied opponents of the philosophes. I argue that the writer Jean-Baptiste-Louis Gresset (1709- 1777), the philosopher Léger-Marie Deschamps (1716-1774), and the lawyer and political theorist Simon-Nicolas-Henri Linguet (1736-1794) opposed the philosophes in the name of a set of universally valid principles against what they took to be the philosophes' superficial, self-serving, and haphazard politicization of language, philosophy and the social sciences. These three intellectuals warned that such politicization fostered economic, political, and intellectual inequality as well as cultural alienation, thereby undermining the Enlightenment’s own vision of a world of self-emancipated human beings and pushing France in the direction of a violent revolution. Gresset supported a “civic republican” political economy of virtue, and warned about the dangers of the consumer culture fostered by the philosophes. The roots of his cultural criticism lay in moral and political concerns that found expression in a patriotic discourse stressing the importance of social “harmony” and the common good while rejecting any temptation to belong to a “party.” In this spirit, Gresset defended the “ancient constitution” against idle monks, royal or ministerial despotism, parlementarian rebellion, and the philosophes. -

Land Tenure in Acadian Agricultural Settlements, 1604-1755: Cultural Retention and the Emergence of Custom Carol A
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library Summer 8-5-2019 Land Tenure in Acadian Agricultural Settlements, 1604-1755: Cultural Retention and the Emergence of Custom Carol A. Blasi University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Part of the Canadian History Commons, Cultural History Commons, and the Legal Commons Recommended Citation Blasi, Carol A., "Land Tenure in Acadian Agricultural Settlements, 1604-1755: Cultural Retention and the Emergence of Custom" (2019). Electronic Theses and Dissertations. 3053. https://digitalcommons.library.umaine.edu/etd/3053 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. LAND TENURE IN ACADIAN AGRICULTURAL SETTLEMENTS, 1604-1755: CULTURAL RETENTION AND THE EMERGENCE OF CUSTOM by Carol Ann Blasi BA, Syracuse University, 1978 MA, The University of Chicago Divinity School, 1981 JD, Temple University School of Law, 1986 A DISSERTATION Submitted in Partial Fulfillment of the Requirements for theDegree of Doctor of Philosophy (in History) The Graduate School The University of Maine August, 2019 Advisory Committee Members: Jacques Ferland, Associate Professor of History, Advisor Alexander Grab, Emeritus Professor of History Stephen J. Hornsby, Professor of Geography and Canadian Studies Richard W. Judd, Emeritus Professor of History Liam Riordan, Professor of History © 2019 Carol Ann Blasi All Rights Reserved ii LAND TENURE IN ACADIAN AGRICULTURAL SETTLEMENTS, 1604-1755 CULTURAL RETENTION AND THE EVOLUTION OF CUSTOM By Carol Ann Blasi Dissertation Advisor: Dr. -

Information Leaflet No.2
The Court of the Lord Lyon Crest Badges Information Leaflet No. 2 Scottish Crest Badges NTRODUCTION Much confusion exists about the meaning, use and entitlement to wear Scottish Crest Badges, and it is constantly increased by well—meaning but ill—informed explanations. This leaflet is authoritative in setting out the main facts. Even the popular name "Clan Crest" is a misnomer, as there is no such thing as a "Clan" Crest. The Crest is the exclusively personal property of the Clan Chief, and it is fully protected to him by the law in Scotland. The circumstances in which it may be worn by his clansmen are set out hereafter. But, first, four brief definitions of the technical terms are necessary. 1. DEFINITIONS (a) THE CREST When a coat of Arms is granted by the Sovereign through Her Majesty’s Lord Lyon King of Arms, the Grant of Arms document shows the coat of Arms on a Shield. Above the Shield is placed a conventional helmet, and on top of the helmet is shown an additional. device called the CREST, accompanied by the owner’s Motto on a conventional scroll. It is impossible to own a Crest without first owning a coat of Arms, shown on the Shield, as the Crest is an adjunct to the coat of Arms. Sometimes an additional Motto or Slogan is granted, which may correctly appear on the Clansman’s bonnet badge as an alternative to the first Motto. (b) THE WREATH Between the Crest and the helmet usually appears a WREATH of twisted cloth of alternate twists of the owner’s "Livery colours", on which the Crest stands. -
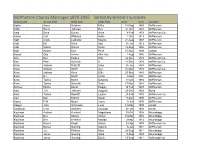
Mcpherson County Marriages 1870-1915 Sorted by Groom's
McPherson County Marriages 1870-1915 Sorted by Groom's Surname Groom Last Groom First Bride Last Bride First Date Year Location Kabler Aaron Delphon Millie 18-May 1889 McPherson Kahn Henry Johnson Eva 21-Oct 1883 McPherson Kaid Band Burley Anna 3-Feb 1879 McPherson Co. Kaiser Carl Williams Nellie 1-Oct 1913 McPherson Kalb Clyde Ledbetter Mayme 21-Aug 1907 McPherson Kalb Ed Rehn Ada 18-Jan 1913 McPherson Kalb Walter Schulz Nellie 12-Sep 1906 McPherson Kalb Walter Dole Pearl 15-Nov 1899 Canton Kampf Otto Achen Allie Von 1-Aug 1880 McPherson Kan Ben Forbes Katy 14-Dec 1879 McPherson Co. Kan Peter Schmidt A. 14-Dec 1879 McPherson Co. Kane Andrew Ratzlaff Anna 25-Jan 1885 McPherson Kane Andrew Smith Avy 16-Nov 1879 McPherson Co. Kane Andrew Kane Effie 25-Mar 1883 McPherson Kane B.J. Smith Lizzie 8-Oct 1891 McPherson Kane Tobias Buller Susanna 13-Oct 1884 McPherson Kane Tobias Schmidt Susie 17-Nov 1884 McPherson Kanney Martin Donat Maggie 19-Feb 1897 McPherson Kant J.W. Johnson Augusta 23-Dec 1902 Elyria Karn Tobias Radcliff Louisa 23-Feb 1879 McPherson Co. Karr Ban Ridcliff Sarah 17-Dec 1880 McPherson Kasey F.W. Moore Jessie 5-Jun 1895 McPherson Kasper John Schroeder Lena 31-May 1900 Lehigh Kauffman Crist Schlatter Amanda 28-Jan 1902 Inman Kaufman Andrew Krehbiel Magdelana 30-Oct 1904 Moundridge Kaufman Ben Stucky Alvina 18-May 1905 Moundridge Kaufman Ben Krehbiel Matilda 2-May 1912 Moundridge Kaufman Daniel Krepil Karlen 23-Apr 1879 McPherson Co. Kaufman Daniel Gehring Katrina 5-Jan 1881 McPherson Kaufman J.A. -

The Chief's Column — Madam Arabella Kincaid Of
THE OFFICIAL NEWSLETTER OF T H E C L A N The Defender K I N C A I D ASSOCIATION INTERNATIONAL THE DEFENDER I S S U E 9 6 – M A R C H 2 0 1 9 INSIDE THIS ISSUE: The Chief’s Column — Madam Arabella Kincaid of Kincaid Chief’s Column 2 continued Fellow Kincaids, Kinsfolk, Friends, President’s 3 Message The very opening words must be addressed to Emily, our Defender Editor, to wish you all the best for the next precious few weeks especially as you welcome a 2019 Gathering 4 new little person into your family! A miracle every time, new life - with all the love, Schedule, Accom- joy and hope that comes with it. modations Greetings from 5 I have just been away, together with Giles and our youngest (Kalula), to the Communica- Durham for a mini break and to see Angus. Whilst we were there we went to an tions Director evensong at the Cathedral. It is hard to find the words to describe Durham Cathe- This and That– 5 dral. The sheer magnificence of the place is overwhelming and the history that we Kincaid can imbibe so easily thanks to the information available is hard to take in in just one 6 visit. I mention this because I was struck forcibly with the impact of what can be Clan Kincaid Gathering Golf achieved, built upon and embellished throughout many generations of people who Shirt Special have invested into a place, family, cause, dynasty etc. The visible structure of the Sarasota Games Cathedral is there for all to see and it has been a place of worship and welcome for In Gratitude for 7 almost a millennium and housed therein is the grave of St Cuthbert (one of the the Gathering most important medieval saints in the North of England with a cult centred on his Membership 8 tomb) and also the Venerable Bede (who is also known as the Father of English His- Update tory). -

Reconstructed Miller (Müller/Möller/Moeller/Mueller
Reconstructed Miller (Müller/Möller/Moeller/Mueller) Families of Freudenberg, Germany From Church Records (Evangelisch, Freudenberg, Westfalen, Prussia) Compiled By Clovis E. Miller This page has been created in the interest of furthering knowledge of our pre-American Miller Family. By this, we can not only establish a foundation for going back further in time; but also create a starting point for researching and linking other members of the German sector of our family, some of which may also have immigrated to America after John Frederick Miller. This page is tentative, being composed primarily of information gleaned from Freudenberg, Germany Church Records; but it is at least a start. In light of this fact, beware of the possibility of children being placed improperly. In most cases the relationship of the father and mother is quite obvious. However, with so many individuals bearing the same first names, and often approximately the birth date time frame, it is quite possible to include an indivudual in the wrong family. There are also no less than four spelling variations used for the Miller lines of Freudenberg. There were a number of Miller (i.e. Müller/Möller/Moeller/Mueller) families in Freudenberg and surrounding towns during the 1600-1800's; including those of the siblings of John Frederick Miller. His sister Elizabeth, for example, married Hymenaeus Creutz (Haman Critz), They immigrated to America with John Frederick and family in 1738 settling in Patrick County, Virgina area. Herman (Harman), one of John's brother, also immigrated at the same time, and eventually settled in Halifax County, Va., near his brother.