Punica Granatum)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Oh Ho Oh Oh Oh Oh O Ho Ch3o N N H O Ho Oh Oh Oh Oh O Ho Ch3o N N
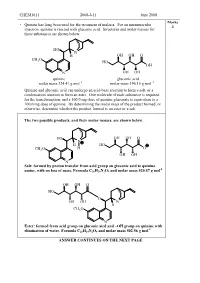
CHEM1611 2008-J-11 June 2008 Marks • Quinine has long been used for the treatment of malaria. For an intramuscular 4 injection, quinine is reacted with gluconic acid. Structures and molar masses for these substances are shown below. HO N H OH OH O CH O 3 HO OH N OH OH quinine gluconic acid molar mass 324.41 g mol –1 molar mass 196.16 g mol –1 Quinine and gluconic acid can undergo an acid-base reaction to form a salt, or a condensation reaction to form an ester. One molecule of each substance is required for the transformation, and a 160.0 mg dose of quinine gluconate is equivalent to a 100.0 mg dose of quinine. By determining the molar mass of the product formed, or otherwise, determine whether the product formed is an ester or a salt. The two possible products, and their molar masses, are shown below. HO OH OH O N H HO CH3O H O OH OH N Salt: formed by proton transfer from acid group on gluconic acid to quinine -1 amine, with no loss of mass. Formula C 26 H26 N2O9 and molar mass 520.57 g mol OH OH O HO O OH OH N H CH3O N Ester: formed from acid group on gluconic aicd and –OH group on quinine with -1 elimination of water. Formula C 26 H24 N2O8 and molar mass 502.56 g mol ANSWER CONTINUES ON THE NEXT PAGE CHEM1611 2008-J-11 June 2008 100.0 mg of quinine corresponds to: ̡̭̳̳ ̊̉̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.083 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̌̋̍.̍̊ ̧ ̭̯̬ ̊ 160.0 mg of the salt product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.074 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̋̉.̎̐ ̧ ̭̯̬ ̊ 160.0 mg of the ester product corresponds to: ̡̭̳̳ ̊̏̉.̉Ɛ̊̉ ̌ ̧ number of moles = Ɣ = 3.184 × 10 -4 mol ̡̭̯̬̲ ̡̭̳̳ ̎̉̋.̎̏ ̧ ̭̯̬ ̊ As the dosages are the same, it must be the salt which is being administered. -

Walnut Polyphenol
ORYZA OIL & FAT CHEMICAL CO., L TD. WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome ■ WALNUT POLYPHENOL-P10,P30 (Powder,Food Grade) ■ WALNUT POLYPHENOL-WSP10 (Water-soluble Powder,Food Grade) ■ WALNUT POLYPHENOL-PC10,PC30 (Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-WSPC10 (Water-soluble Powder,Cosmetic Grade) ■ WALNUT POLYPHENOL-LC (Water-soluble Liquid,Cosmetic Grade) ■ WALNUT SEED OIL (Oil,Food & Cosmetic Grade) ORYZA OIL & FAT CHEMICAL CO., LTD ver. 1.0 HS WALNUT POLYPHENOL ver.1.0 HS WALNUT POLYPHENOL Hepatoprotective & Anti-oxidative Extract For Metabolic Syndrome 1. Introduction Recently, there is an increased awareness on metabolic syndrome – a condition characterized by a group of metabolic risk factors in one person. They include abdominal obesity, atherogenic dyslipidemia, elevated blood pressure, insulin resistance, prothrombotic state & proinflammatory state. The dominant underlying risk factors appear to be abdominal obesity and insulin resistance. In addition, non-alcoholic fatty liver disease (NAFLD) is the most commonly associated “liver” manifestation of metabolic syndrome which can progress to advance liver disease (e.g. cirrhosis) with associated morbidity and mortality. Lifestyle therapies such as weight loss significantly improve all aspects of metabolic syndrome, as well as reducing progression of NAFLD and cardiovascular mortality. Walnut (Juglans regia L. seed) is one the most popular nuts consumed in the world. It is loaded in polyunsaturated fatty acids – linoleic acid (LA), oleic acid and α-linolenic acid (ALA), an ω3 fatty acid. It has been used since ancient times and epidemiological studies have revealed that incorporating walnuts in a healthy diet reduces the risk of cardiovascular diseases. Recent investigations reported that walnut diet improves the function of blood vessels and lower serum cholesterol. -

(12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski Et Al
USOO9421 180B2 (12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski et al. (45) Date of Patent: Aug. 23, 2016 (54) ANTIOXIDANT COMPOSITIONS FOR 6,203,817 B1 3/2001 Cormier et al. .............. 424/464 TREATMENT OF INFLAMMATION OR 6,323,232 B1 1 1/2001 Keet al. ............ ... 514,408 6,521,668 B2 2/2003 Anderson et al. ..... 514f679 OXIDATIVE DAMAGE 6,572,882 B1 6/2003 Vercauteren et al. ........ 424/451 6,805,873 B2 10/2004 Gaudout et al. ....... ... 424/401 (71) Applicant: Perio Sciences, LLC, Dallas, TX (US) 7,041,322 B2 5/2006 Gaudout et al. .............. 424/765 7,179,841 B2 2/2007 Zielinski et al. .. ... 514,474 (72) Inventors: Jan Zielinski, Vista, CA (US); Thomas 2003/0069302 A1 4/2003 Zielinski ........ ... 514,452 Russell Moon, Dallas, TX (US); 2004/0037860 A1 2/2004 Maillon ...... ... 424/401 Edward P. Allen, Dallas, TX (US) 2004/0091589 A1 5, 2004 Roy et al. ... 426,265 s s 2004/0224004 A1 1 1/2004 Zielinski ..... ... 424/442 2005/0032882 A1 2/2005 Chen ............................. 514,456 (73) Assignee: Perio Sciences, LLC, Dallas, TX (US) 2005, 0137205 A1 6, 2005 Van Breen ..... 514,252.12 2005. O154054 A1 7/2005 Zielinski et al. ............. 514,474 (*) Notice: Subject to any disclaimer, the term of this 2005/0271692 Al 12/2005 Gervasio-Nugent patent is extended or adjusted under 35 et al. ............................. 424/401 2006/0173065 A1 8/2006 BeZwada ...................... 514,419 U.S.C. 154(b) by 19 days. 2006/O193790 A1 8/2006 Doyle et al. -

Punicalin Alleviates OGD/R-Triggered Cell Injury Via TGF-Β-Mediated Oxidative Stress and Cell Cycle in Neuroblastoma Cells SH-SY5Y
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2021, Article ID 6671282, 11 pages https://doi.org/10.1155/2021/6671282 Research Article Punicalin Alleviates OGD/R-Triggered Cell Injury via TGF-β-Mediated Oxidative Stress and Cell Cycle in Neuroblastoma Cells SH-SY5Y Tiansong Yang,1 Qingyong Wang,2 Yuanyuan Qu,2 Yan Liu,1 Chuwen Feng,1 Yulin Wang,2 Weibo Sun,3 Zhongren Sun ,2 and Yulan Zhu4 1First affiliated hospital, Heilongjiang University of Chinese Medicine, Harbin, China 2Heilongjiang University of Chinese Medicine, Harbin, China 3Harbin Medical University, Harbin, China 4Department of Neurology, %e Second Affiliated Hospital of Harbin Medical University, Harbin, China Correspondence should be addressed to Zhongren Sun; [email protected] Received 21 October 2020; Revised 21 October 2020; Accepted 7 January 2021; Published 12 February 2021 Academic Editor: Muhammad Farrukh Nisar Copyright © 2021 Tiansong Yang et al. /is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. /e research aimed to identify the active component from Punica granatum L. to alleviate ischemia/reperfusion injury and clarify the underlying mechanism of the active component alleviating ischemia/reperfusion injury. Materials and Methods. /e SH-SY5Y cell model of oxygen-glucose deprivation/reoxygenation (OGD/R) was established to simulate the ischemia/ reperfusion injury. According to the strategy of bioassay-guided isolation, the active component of punicalin from Punica granatum L. was identified. Flow cytometry and Western blotting were employed to evaluate the effects of OGD/R and/or punicalin on cell cycle arrest. -

Punica Granatum L
Research Article Studies on antioxidant activity of red, white, and black pomegranate (Punica granatum L.) peel extract using DPPH radical scavenging method Uswatun Chasanah[1]* 1 Department of Pharmacy, Faculty of Health Science, University of Muhammadiyah Malangg, Malang, East Java, Indonesia * Corresponding Author’s Email: [email protected] ARTICLE INFO ABSTRACT Article History Pomegranate (Punica granatum L.) has high antioxidant activity. In Received September 1, 2020 Indonesia, there are red pomegranate, white pomegranate, and black Revised January 7, 2021 pomegranate. The purpose of this study was to determine the antioxidant Accepted January 14, 2021 activity of red pomegranate peel extract, white pomegranate peel extract, Published February 1, 2021 and black pomegranate peel extract. The extracts prepared by ultrasonic maceration in 96% ethanol, then evaporated until thick extract was Keywords obtained and its antioxidant activity was determined using the DPPH Antioxidant radical scavenging method. This study showed that all pomegranate peel Black pomegranate extract varieties have potent antioxidant activity and the black Red pomegranate pomegranate peel extract has the highest antioxidant power. White pomegranate Peel extract DPPH Doi 10.22219/farmasains.v5i2.13472 1. INTRODUCTION Pomegranate (Punica granatum L.) belongs to the Puricaceae family, a plant originating from the Middle East (Rana, Narzary & Ranade, 2010). All parts of the pomegranate, such as fruit (fruit juice, fruit seeds, peel fruit), leaves, flowers, roots, and bark, have therapeutic effects such as neuroprotective, antioxidant, repair vascular damage, and anti-inflammatory. The clinical application of this plant used in cancers, atherosclerosis, hyperlipidemia, carotid artery stenosis, myocardial perfusion, periodontal disease, bacterial infections, ultraviolet radiation, erectile dysfunction, male infertility, neonatal hypoxic-ischemic brain injury, Alzheimer's disease, and obesity (Jurenka, 2008; Mackler, Heber & Cooper, 2013). -

A Combined Experimental and Computational Study of Chrysanthemin As a Pigment for Dye-Sensitized Solar Cells
molecules Article A Combined Experimental and Computational Study of Chrysanthemin as a Pigment for Dye-Sensitized Solar Cells Atoumane Ndiaye 1,2 , Alle Dioum 1, Corneliu I. Oprea 2 , Anca Dumbrava 3,* , Jeanina Lungu 2, Adrian Georgescu 2, Florin Moscalu 2, Mihai A. Gîr¸tu 2,* , Aboubaker Chedikh Beye 1 and Issakha Youm 1,* 1 Department of Physics, Cheikh Anta Diop University of Dakar, 5005 Dakar-Fann, Senegal; [email protected] (A.N.); [email protected] (A.D.); [email protected] (A.C.B.) 2 Department of Physics, Ovidius University of Constanta, 900527 Constanta, Romania; [email protected] (C.I.O.); [email protected] (J.L.); [email protected] (A.G.); fl[email protected] (F.M.) 3 Department of Chemistry and Chemical Engineering, Ovidius University of Constanta, 900527 Constanta, Romania * Correspondence: [email protected] (A.D.); [email protected] (M.A.G.); [email protected] (I.Y.) Abstract: The theoretical study of chrysanthemin (cyanidin 3-glucoside) as a pigment for TiO2-based dye-sensitized solar cells (DSSCs) was performed with the GAUSSSIAN 09 simulation. The electronic spectra of neutral and anionic chrysanthemin molecules were calculated by density functional theory with B3LYP functional and DGDZVP basis set. A better energy level alignment was found for partially deprotonated molecules of chrysanthemin, with the excited photoelectron having enough energy in order to be transferred to the conduction band of TiO2 semiconductor in DSSCs. In addition, we used the raw aqueous extracts of roselle (Hibiscus sabdariffa) calyces as the source of chrysanthemin Citation: Ndiaye, A.; Dioum, A.; and the extracts with various pH values were tested in DSSCs. -

Plant Phenolics: Bioavailability As a Key Determinant of Their Potential Health-Promoting Applications
antioxidants Review Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications Patricia Cosme , Ana B. Rodríguez, Javier Espino * and María Garrido * Neuroimmunophysiology and Chrononutrition Research Group, Department of Physiology, Faculty of Science, University of Extremadura, 06006 Badajoz, Spain; [email protected] (P.C.); [email protected] (A.B.R.) * Correspondence: [email protected] (J.E.); [email protected] (M.G.); Tel.: +34-92-428-9796 (J.E. & M.G.) Received: 22 October 2020; Accepted: 7 December 2020; Published: 12 December 2020 Abstract: Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom that can be categorized as flavonoids and non-flavonoids. Interest in phenolic compounds has dramatically increased during the last decade due to their biological effects and promising therapeutic applications. In this review, we discuss the importance of phenolic compounds’ bioavailability to accomplish their physiological functions, and highlight main factors affecting such parameter throughout metabolism of phenolics, from absorption to excretion. Besides, we give an updated overview of the health benefits of phenolic compounds, which are mainly linked to both their direct (e.g., free-radical scavenging ability) and indirect (e.g., by stimulating activity of antioxidant enzymes) antioxidant properties. Such antioxidant actions reportedly help them to prevent chronic and oxidative stress-related disorders such as cancer, cardiovascular and neurodegenerative diseases, among others. Last, we comment on development of cutting-edge delivery systems intended to improve bioavailability and enhance stability of phenolic compounds in the human body. Keywords: antioxidant activity; bioavailability; flavonoids; health benefits; phenolic compounds 1. Introduction Phenolic compounds are secondary metabolites widely spread throughout the plant kingdom with around 8000 different phenolic structures [1]. -

A Review on Antihyperglycemic and Antihepatoprotective Activity of Eco-Friendly Punica Granatum Peel Waste
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2013, Article ID 656172, 10 pages http://dx.doi.org/10.1155/2013/656172 Review Article A Review on Antihyperglycemic and Antihepatoprotective Activity of Eco-Friendly Punica granatum Peel Waste Sushil Kumar Middha,1 Talambedu Usha,2 and Veena Pande1 1 Department of Biotechnology, Bhimtal Campus, Kumaun University, Nainital, Uttarakhand 263136, India 2 Department of Biotechnology & Biochemistry, Maharani Lakshmi Ammanni College for Women, Bangalore 560012, India Correspondence should be addressed to Veena Pande; veena [email protected] Received 28 December 2012; Revised 25 March 2013; Accepted 25 April 2013 Academic Editor: Edwin L. Cooper Copyright © 2013 Sushil Kumar Middha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Over the past decade, pomegranate (Punica granatum) is entitled as a wonder fruit because of its voluminous pharmacological properties. In 1830, P. g ranatum fruit was first recognized in United States Pharmacopeia; the Philadelphia edition introduced the rind of the fruit, the New York edition the bark of the root and further 1890 edition the stem bark was introduced. There are significant efforts and progress made in establishing thepharmacological mechanisms of peel (pericarp or rind) and the individual constituents responsible for them. This review provides an insight on the phytochemical components that contribute too antihyperglycemic, hepatoprotective, antihyperlipidemic effect, and numerous other effects of wonderful, economic, and eco- friendly pomegranate peel extract (PP). 1. Introduction containing sacs packed with a fleshy, juicy, red or whitish pulp. -

Namenverzeichnis. Index of Names. Index Des Auteurs
Namenverzeichnis. Index of Names. Index des Auteurs. ABDERHALDEN, E. 398, 400. ARNOLD, ,V. 200, 201, 236, 237, 241. ABELSON, P. H. 379, 383, 389, 390, 393, ARONOFF, S. 26, 59, 258, 282. 394, 395, 400, 402. ASAHINA, Y. 13, 130, 131 , 175. ADAIR, G. S. 327, 371. ASANO, J. 131, 175· ADAMS, R. 147, 149, 175. ASHBY, ''I'. C. 267, 282. ADANK, K. 189, 237, 238, 241, 242, 245. ASHWORTH, B. DE 49, 61. ADRIAN, M. M. 138, 175. ASMIS, H. 190, 2lI, 214, 215, 228, 236, AGREN, G. 383, 400. 238, 241. AHLUWALIA, V. K. 13, 21, 40, 59. Aso, K. 175. AHMED, M. 173, 178. ASTON, B. C. 161, 177. AHRENS, G. 84, 118. AusKAPS, J. 434, 446. AKABORI, S. 360, 371. AVERY, G. S., Jr. 258, 270, 273, 275, ALADASHVILI, V. A. 127, 175. 276, 282, 283. ALDAG, H. J. 434, 448. ALDER, K. 77, 8o, lI8, 154, 175. BACCARINI, P. 252, 283. ALDERWEIRELDT, F. 165. BACHLI, E. 190, 204, 207, 2I5, 225, 226, ALGAR, J. 41, 59. 228, 236, 238, 239, 241, 242, 243· ALLAN, J. 34, 59· BACHMANN, E. 312, 313, 3 18. ALLEN, D. W. 332, 338, 340, 344, 345, BADER, F. E. 191, 202, 239, 241. 347, 348, 349, 350, 35 1, 352, 368 371, BAHR, K. 18 5, 187, 189, 207, 2II, 2I5, 372. 218, 219, 220, 236, 237, 238, 246. ALLEN, F. 127, 175. 'BAKER, J. W. 281, 283. ALLEN, P. W. 420, 425, 445. II BAKER, W. 3, 16, 35, 41, 43, 44, 56, 59· ALLISON, A. C. 334, 356, 372. -

Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacte
S. Dinç et al. / Hacettepe J. Biol. & Chem., 2017, 45 (4), 603-607 Promising Strain of Acinetobacter from Soil for Utilization of Gluconic Acid Production Topraktaki Gelecek Vaadeden Acinetobacter Suşunun Glukonik Asit Üretiminde Kullanımı Research Article Saliha Dinç 1,2*, Meryem Kara2,3, Mehmet Öğüt3, Fatih Er1, Hacer Çiçekçi2 1Selcuk University Cumra School of Applied Sciences, Konya-Turkey. 2Selcuk Uni. Advanced Technology Research and Application Center, Konya-Turkey. 3Selcuk University Cumra Vocational High School, Konya-Turkey. ABSTRACT luconic acid, a food additive, is used in many foods to control acidity or binds metals such as calcium, Giron. Acinetobacter sp. WR326, newly isolated from soil possesses high phosphate solubilizing activity and do not require pyrroloquinoline quinone (PQQ) for glucose dehydrogenase (GDH) activity as cofactor In this study the gluconic acid production potential of this bacterium was investigated. Firstly, Acinetobacter sp. WR326 was incubated in tricalcium phosphate medium (TCP) with varying glucose concentrations (100, 250, 500 mM), at a temperature of 30°C for 5 days (120 hours). The highest gluconic acid yield (59%) was found at a glucose concentration of 100 mM. Then three different levels of gluconic acid addition to the medium (50, 100, 200 mM) were tested. When Acinetobacter sp. WR326 strain was cultivated with a 100 mM glucose and 100 mM gluconic acid the yield increased to 95.27%. In any trials 2 -keto D-gluconic acid, causes problems in processing and purification of the gluconic acid, was not detected in the medium. As a conclusion, Acinetobac- ter sp. WR326 may be considered novel potential bacterial strain for gluconic acid production. -

(12) United States Patent (10) Patent No.: US 7,919,636 B2 Seeram Et Al
USOO7919636B2 (12) United States Patent (10) Patent No.: US 7,919,636 B2 Seeram et al. (45) Date of Patent: Apr. 5, 2011 (54) PURIFICATIONS OF POMEGRANATE Aviram, M., et al., “Pomegranate juice consumption inhibits serum ELLAGTANNINS AND THEIR USES angiotensin converting enzyme activity and reduces systolic blood THEREOF pressure.” (2001) Atherosclerosis, 158: 195-198. Cerda, B., et al., “Evaluation of bioavailability and metabolism in the (75) Inventors: Navindra P. Seeram, Los Angeles, CA rat of punicalagin, an antioxidant polyphenol from pomegranate juice.” (2003) Eur, J. Nutr., 42:18-28. (US); David Heber, Los Angeles, CA Cerda, B., et al., “Repeated oral administration of high doses of the (US) pomegranate elagitannin punicalaginto rats for 37 days is not toxic.” (2003) J. Agric. Food Chem. 51:3493-3501. (73) Assignee: The Regents of the University of Doig, A., et al., “Isolation and structure elucidation of punicalagin, a California, Oakland, CA (US) toxic hydrolysable tannin, from Terminalia oblongata.” (1990) J. Chem. Soc. Perkin Trans. I, 2317-2321. (*) Notice: Subject to any disclaimer, the term of this El-Toumy, S., et al., “Two ellagitannins from Punica granatum patent is extended or adjusted under 35 heartwood.” (2002) Phytochemistry, 61:971-974. U.S.C. 154(b) by 248 days. Filippich, L., et al., “Hepatotoxic and nephrotoxic principles in Terminalia oblongata.” (1991) Research in Veterinary Science, (21) Appl. No.: 12/143,657 50:17O-177. Gil, M., et al., “Antioxidant activity of pomegranate juice and its (22) Filed: Jun. 20, 2008 relationship with phenolic composition and processing.” (2000) J. Agric. Food Chem., 48:4581-4589. -

Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy
molecules Article Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy Valtteri Virtanen * , Susanna Räikkönen, Elina Puljula and Maarit Karonen Natural Chemistry Research Group, Department of Chemistry, University of Turku, FI-20014 Turku, Finland; [email protected] (S.R.); [email protected] (E.P.); maarit.karonen@utu.fi (M.K.) * Correspondence: vtjvir@utu.fi; Tel.: +358-29-450-3205 Abstract: Ellagitannins have antimicrobial activity, which might be related to their interactions with membrane lipids. We studied the interactions of 12 different ellagitannins and pentagalloylglucose with a lipid extract of Escherichia coli by high-resolution magic angle spinning NMR spectroscopy. The nuclear Overhauser effect was utilized to measure the cross relaxation rates between ellagitannin and lipid protons. The shifting of lipid signals in 1H NMR spectra of ellagitannin–lipid mixture due to ring current effect was also observed. The ellagitannins that showed interaction with lipids had clear structural similarities. All ellagitannins that had interactions with lipids had glucopy- ranose cores. In addition to the central polyol, the most important structural feature affecting the interaction seemed to be the structural flexibility of the ellagitannin. Even dimeric and trimeric ellagitannins could penetrate to the lipid bilayers if their structures were flexible with free galloyl and hexahydroxydiphenoyl groups. Keywords: E. coli; HR-MAS-NMR; interaction; lipid membrane; tannins; UPLC-DAD-MS Citation: Virtanen, V.; Räikkönen, S.; Puljula, E.; Karonen, M. 1. Introduction Ellagitannin–Lipid Interaction by HR-MAS NMR Spectroscopy. Tannins are a group of specialized plant metabolites, which, when included in the di- Molecules 2021, 26, 373. etary feed of ruminants, have been shown to induce many beneficial effects such as increas- https://doi.org/10.3390/ ing their effective amino acid absorption, lowering their methane production, and acting as molecules26020373 anthelmintics [1–6].