The Stream Biome Gradient Concept: Factors Controlling Lotic Systems Across Broad Biogeographic Scales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characteristics and Importance of Rill and Gully Erosion: a Case Study in a Small Catchment of a Marginal Olive Grove
Cuadernos de Investigación Geográfica 2015 Nº 41 (1) pp. 107-126 ISSN 0211-6820 DOI: 10.18172/cig.2644 © Universidad de La Rioja CHARACTERISTICS AND IMPORTANCE OF RILL AND GULLY EROSION: A CASE STUDY IN A SMALL CATCHMENT OF A MARGINAL OLIVE GROVE E.V. TAGUAS1*, E. GUZMÁN1, G. GUZMÁN1, T. VANWALLEGHEM1, J.A. GÓMEZ2 1Rural Engineering Department/ Agronomy Department, University of Cordoba, Campus Rabanales, Leonardo Da Vinci building, 14071 Córdoba, Spain. 2Institute for Sustainable Agriculture, CSIC, Apartado 4084, 14080 Córdoba, Spain. ABSTRACT. Measurements of gullies and rills were carried out in an olive or- chard microcatchment of 6.1 ha over a 4-year period (2010-2013). No tillage management allowing the development of a spontaneous grass cover was imple- mented in the study period. Rainfall, runoff and sediment load were measured at the catchment outlet. The objectives of this study were: 1) to quantify erosion by concentrated flow in the catchment by analysis of the geometric and geomor- phologic changes of the gullies and rills between July 2010 and July 2013; 2) to evaluate the relative percentage of erosion derived from concentrated runoff to total sediment yield; 3) to explain the dynamics of gully and rill formation based on the hydrological patterns observed during the study period; and 4) to improve the management strategies in the olive grove. Control sections in gullies were established in order to get periodic measurements of width, depth and shape in each campaign. This allowed volume changes in the concentrated flow network to be evaluated over 3 periods (period 1 = 2010-2011; period 2 = 2011-2012; and period 3 = 2012-2013). -

Alluvial Fans in the Death Valley Region California and Nevada
Alluvial Fans in the Death Valley Region California and Nevada GEOLOGICAL SURVEY PROFESSIONAL PAPER 466 Alluvial Fans in the Death Valley Region California and Nevada By CHARLES S. DENNY GEOLOGICAL SURVEY PROFESSIONAL PAPER 466 A survey and interpretation of some aspects of desert geomorphology UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1965 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director The U.S. Geological Survey Library has cataloged this publications as follows: Denny, Charles Storrow, 1911- Alluvial fans in the Death Valley region, California and Nevada. Washington, U.S. Govt. Print. Off., 1964. iv, 61 p. illus., maps (5 fold. col. in pocket) diagrs., profiles, tables. 30 cm. (U.S. Geological Survey. Professional Paper 466) Bibliography: p. 59. 1. Physical geography California Death Valley region. 2. Physi cal geography Nevada Death Valley region. 3. Sedimentation and deposition. 4. Alluvium. I. Title. II. Title: Death Valley region. (Series) For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C., 20402 CONTENTS Page Page Abstract.. _ ________________ 1 Shadow Mountain fan Continued Introduction. ______________ 2 Origin of the Shadow Mountain fan. 21 Method of study________ 2 Fan east of Alkali Flat- ___-__---.__-_- 25 Definitions and symbols. 6 Fans surrounding hills near Devils Hole_ 25 Geography _________________ 6 Bat Mountain fan___-____-___--___-__ 25 Shadow Mountain fan..______ 7 Fans east of Greenwater Range___ ______ 30 Geology.______________ 9 Fans in Greenwater Valley..-----_____. 32 Death Valley fans.__________--___-__- 32 Geomorpholo gy ______ 9 Characteristics of fans.._______-___-__- 38 Modern washes____. -

SCI Lecture Paper Series
SCI LECTURE PAPERS SERIES HIGHWAY DRAINAGE SYSTEMS Santi V Santhalingam Highways Agency, Room 4/41, St. Christopher House Southwark Street, London SE1 0TE Telephone +44 (0) 171 921 4954 Fax +44 (0) 171 921 4411 © Highways Agency 1999 copyright reserved ISSN 1353-114X LPS 102/99 Key words highways, drainage, runoff, surface, sub-surface, systems INTRODUCTION Appropriate drainage is an important feature of good highway design in terms of ensuring required level of service and value for money are achieved. Highway drainage has two major objectives: safety of the road user and longevity of the pavement. Speedy removal of surface water will help to ensure safe and comfortable conditions for the road user. Provision of effective sub-drainage will maximise longevity of the pavement and its associated earthworks. Highway drainage can therefore be broadly classified into two elements – surface run-off and sub-surface run-off: these two elements are not completely disparate in that some of the surface water may find its way into the road foundation through surfaces which are not completely impermeable thence requiring removal by sub-drainage. Based on these fundamental principles, drainage methods in the UK are broadly divided into two categories: (a) combined systems, where the surface and sub-surface water are collected and transported in the same pipe, and (b) separate systems, where the two elements are collected and transported in separate pipes Within the broader definition of the two systems there are a number of different drainage methods that are in use on UK highways, some of them more common than others. -

Erosion-1.Pdf
R E S O U R C E L I B R A R Y E N C Y C L O P E D I C E N T RY Erosion Erosion is the geological process in which earthen materials are worn away and transported by natural forces such as wind or water. G R A D E S 6 - 12+ S U B J E C T S Earth Science, Geology, Geography, Physical Geography C O N T E N T S 9 Images For the complete encyclopedic entry with media resources, visit: http://www.nationalgeographic.org/encyclopedia/erosion/ Erosion is the geological process in which earthen materials are worn away and transported by natural forces such as wind or water. A similar process, weathering, breaks down or dissolves rock, but does not involve movement. Erosion is the opposite of deposition, the geological process in which earthen materials are deposited, or built up, on a landform. Most erosion is performed by liquid water, wind, or ice (usually in the form of a glacier). If the wind is dusty, or water or glacial ice is muddy, erosion is taking place. The brown color indicates that bits of rock and soil are suspended in the fluid (air or water) and being transported from one place to another. This transported material is called sediment. Physical Erosion Physical erosion describes the process of rocks changing their physical properties without changing their basic chemical composition. Physical erosion often causes rocks to get smaller or smoother. Rocks eroded through physical erosion often form clastic sediments. -

Sustainable Drainage Systems Maximising the Potential for People and Wildlife
The RSPB is the UK charity working to secure a healthy environment for 978-1-905601-41-7 birds and wildlife, helping to create a better world for us all. Our Conservation Management Advice team works to improve the conservation status of priority habitats and species by promoting best- practice advice to land managers. www.rspb.org.uk The Wildfowl & Wetlands Trust (WWT) is one of the world’s largest and most respected wetland conservation organisations working globally to safeguard and improve wetlands for wildlife and people. Founded in 1946 by the late Sir Peter Scott, WWT also operates a unique UK-wide network of specialist wetland centres that protect over 2,600 hectares of important wetland habitat and inspire people to connect with and value wetlands and their wildlife. All of our work is supported by a much valued membership base of over 200,000 people. WWT’s mission is to save wetlands for wildlife and for people and we will achieve this through: • Inspiring people to connect with and value wetlands and their wildlife. • Demonstrating and promoting the importance and benefits of wetlands. • Countering threats to wetlands. • Creating and restoring wetlands and protecting key wetland sites. • Saving threatened wetland species. www.wwt.org.uk Supported by: Sustainable drainage systems Maximising the potential for people and wildlife A guide for local authorities and developers The Royal Society for the Protection of Birds (RSPB) is a registered charity: England & Wales no. 207076, Scotland no. SC037654 The Wildfowl & Wetlands Trust Limited is a charity (1030884 England and Wales, SC039410 Scotland) and a company limited by guarantee (2882729 England). -

Erosion and Sediment Transport Modelling in Shallow Waters: a Review on Approaches, Models and Applications
International Journal of Environmental Research and Public Health Review Erosion and Sediment Transport Modelling in Shallow Waters: A Review on Approaches, Models and Applications Mohammad Hajigholizadeh 1,* ID , Assefa M. Melesse 2 ID and Hector R. Fuentes 3 1 Department of Civil and Environmental Engineering, Florida International University, 10555 W Flagler Street, EC3781, Miami, FL 33174, USA 2 Department of Earth and Environment, Florida International University, AHC-5-390, 11200 SW 8th Street Miami, FL 33199, USA; melessea@fiu.edu 3 Department of Civil Engineering and Environmental Engineering, Florida International University, 10555 W Flagler Street, Miami, FL 33174, USA; fuentes@fiu.edu * Correspondence: mhaji002@fiu.edu; Tel.: +1-305-905-3409 Received: 16 January 2018; Accepted: 10 March 2018; Published: 14 March 2018 Abstract: The erosion and sediment transport processes in shallow waters, which are discussed in this paper, begin when water droplets hit the soil surface. The transport mechanism caused by the consequent rainfall-runoff process determines the amount of generated sediment that can be transferred downslope. Many significant studies and models are performed to investigate these processes, which differ in terms of their effecting factors, approaches, inputs and outputs, model structure and the manner that these processes represent. This paper attempts to review the related literature concerning sediment transport modelling in shallow waters. A classification based on the representational processes of the soil erosion and sediment transport models (empirical, conceptual, physical and hybrid) is adopted, and the commonly-used models and their characteristics are listed. This review is expected to be of interest to researchers and soil and water conservation managers who are working on erosion and sediment transport phenomena in shallow waters. -

Optioservus Riffle Beetle
Table of Contents I. INTRODUCTION............................................................................................................................... 2 II. SPECIES ACCOUNT......................................................................................................................... 2 A. TAXONOMY DESCRIPTION .............................................................................................................. 2 1. Original Description ................................................................................................................... 2 2. Taxonomic Description ............................................................................................................... 4 B. HISTORICAL AND CURRENT DISTRIBUTION ................................................................................... 5 1. Description of habitats and locations of occurrence................................................................... 5 C. POPULATION SIZES AND ABUNDANCE ............................................................................................. 8 D. REPRODUCTIVE HABITS, HABITATS, REQUIREMENTS, AND STRATEGIES..................................... 11 E. FOOD AND FEEDING REQUIREMENTS ............................................................................................ 12 F. OTHER PERTINENT INFORMATION AND SUMMARY ...................................................................... 12 III. OWNERSHIP OF SPECIES HABITAT BY COUNTY........................................................... 13 IV. -

TAHQUITZ CREEK TRAIL MASTER PLAN Background, Goals and Design Standards Tahquitz Creek Trail Master Plan
TAHQUITZ CREEK T RAIL MASTER PLAN PREPARED FOR: THE CITY OF PALM SPRINGS PARKS & RECREATION DEPARTMENT PREPARED BY: ALTA PLANNING + DESIGN WITH RBF CONSULTING MARCH 2010 ACKNOWLEDGEMENTS Deep appreciati on to the neighborhood groups and community members who conti nue to work ti relessly to bring the vision of the Tahquitz Creek Trail to fruiti on. Steering Committ ee Members Council Member Ginny Foat April Hildner Jim Lundin Bill Post Max Davila Lauri Aylaian Steve Sims Mike Hutchison Renee Cain Nanna D. A. Nanna Sharon Heider, Director City of Palm Springs Department of Parks and Recreati on 401 South Pavilion Way P.O. Box 2743 Palm Springs, CA 922-2743 George Hudson, Principal Karen Vitkay, Project Manager Alta Planning + Design, Inc. 711 SE Grand Avenue Portland, Oregon 97214 www.altaplanning.com RBF Consulti ng Brad Mielke, S.E., P.E. 74-130 Country Club Drive, Suite 201 Palm Desert, CA 92260-1655 www.RBF.com TABLE OF CONTENTS Background, Goals and Design and Standards .............................................1 Background ...................................................................................................... 2 Vision Statement .............................................................................................. 2 Goals and Objecti ves ........................................................................................ 3 Trail Design Standards .......................................................................................4 Multi -Use Trail Design ...................................................................................... -
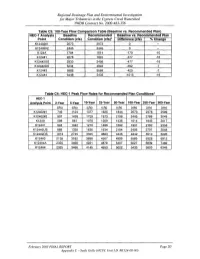
Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No
Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No. 2000-483-356 Table C3: 100-Year Flow Comparison Table (Baseline vs. Recommended Plan) HEC-1 Analysis Baseline Recommended Baseline vs. Recommended Plan Point Condition (cfs) Condition (cfs)· Difference (cfs) "10 Change K12402#1 2073 2073 0 -- K12402#2 2445 2445 0 -- K124A 1784 1614 -170 -10 K124#1 2278 1901 -377 -16 K124#2US 2933 2456 -477 -16 K124#2DS 5234 4842 -392 -7 K124#3 5989 5569 -420 -7 K124#4 6448 5433 -1015 -16 Table C4' HEC-1 Peak Flow Rates for Recommended Plan Conditions· HEC-1 Analysis Point 2-Year S-Year 10-Year 2S-Year SO-Year 100-Year 2S0-Year SOO-Year (cis) (cis) (cis) (cis) (cis) (cis) (cis) ~cls) K12402#1 746 1124 1377 1625 1844 2073 2376 2599 K12402#2 937 1428 1729 1973 2199 2445 2788 3049 K124A 588 881 1076 1269 1436 1614 1845 2017 K124#1 694 1042 1270 1496 1692 1901 2162 2358 K124#2US 889 1339 1636 1934 2184 2456 2797 3048 K124#2DS 1813 2745 3355 3883 4346 4842 5510 6026 K124#3 2136 3182 3898 4507 4999 5569 6328 6912 K124#4A 2325 3466 4221 4878 5407 6027 6839 7462 K124#4 2325 3466 4145 4653 5022 5433 5953 6349 February 2003 FINAL REPORT Page 20 Appendix C - Seals Gully (HCFC Unit I.D. #KI24-00-00) Regional Drainage Plan and Environmental Investigation for Major Tributaries in the Cypress Creek Watershed TWDB Contract No. 2000-483-356 Table C5: Comparison of Water Surface Elevations (100-Year) Seals Gully (K124-00-00' Baseline Condition Recommended Plan Difference Station Location Flow WSEL -

Rill Erosion on an Oxisol Influenced by a Thin Compacted Layer 1383
RILL EROSION ON AN OXISOL INFLUENCED BY A THIN COMPACTED LAYER 1383 Nota RILL EROSION ON AN OXISOL INFLUENCED BY A THIN COMPACTED LAYER(1) Edivaldo Lopes Thomaz(2) SUMMARY The presence of compacted layers in soils can induce subprocesses (e.g., discontinuity of water flow) and induces soil erosion and rill development. This study assesses how rill erosion in Oxisols is affected by a plow pan. The study shows that changes in hydraulic properties occur when the topsoil is eroded because the compacted layer lies close below the surface. The hydraulic properties that induce sediment transport and rill formation (i.e., hydraulic thresholds at which these processes occur) are not the same. Because of the resistance of the compacted layer, the hydraulic conditions leading to rill incision on the soil surface differed from the conditions inducing rill deepening. The Reynolds number was the best hydraulic predictor for both processes. The formed rills were shallow and could easily be removed by tillage between crops. However, during rill development, large amounts of soil and contaminants could also be transferred. Index terms: conventional tillage, plow-pan, hydropedology, surface runof, rill erosion. RESUMO: EROSÃO EM RAVINA EM UM LATOSSOLO INFLUENCIADO POR UMA CAMADA COMPACTADA RASA A presença de uma camada compactada no solo pode induzir a subprocessos como a descontinuidade hidráulica e impor outros limiares para a erosão dele e o desenvolvimento de ravina. Neste estudo foi avaliado como a erosão em ravina em um Latossolo é influenciada por um pé-de-grade. No estudo ficou demonstrado que ocorre mudança nas variáveis hidráulicas quando o topo do solo é erodido e a camada compactada fica próxima da superfície. -

Technical Supplement 14P--Gullies and Their Control
Technical Gullies and Their Control Supplement 14P (210–VI–NEH, August 2007) Technical Supplement 14P Gullies and Their Control Part 654 National Engineering Handbook Issued August 2007 Cover photo: Gully erosion may be a significant source of sediment to the stream. Gullies may also form in the streambanks due to uncontrolled flows from the flood plain (valley trenches). Advisory Note Techniques and approaches contained in this handbook are not all-inclusive, nor universally applicable. Designing stream restorations requires appropriate training and experience, especially to identify conditions where various approaches, tools, and techniques are most applicable, as well as their limitations for design. Note also that prod- uct names are included only to show type and availability and do not constitute endorsement for their specific use. (210–VI–NEH, August 2007) Technical Gullies and Their Control Supplement 14P Contents Purpose TS14P–1 Introduction TS14P–1 Classical gullies and ephemeral gullies TS14P–3 Gullying processes in streams TS14P–3 Issues contributing to gully formation or enlargement TS14P–5 Land use practices .........................................................................................TS14P–5 Soil properties ................................................................................................TS14P–6 Climate ............................................................................................................TS14P–6 Hydrologic and hydraulic controls ..............................................................TS14P–6 -

Driving Directions Lower Yakima MP 19 PLEASE READ the ENTIRE MESSAGE
Driving Directions Lower Yakima MP 19 PLEASE READ THE ENTIRE MESSAGE. If you have questions after reading this email don’t hesitate to contact us. (509)964-2530 • [email protected] Before Your Rafting Trip: 1. Check the weather and dress appropriately (avoid cotton on cold days) 2. Bring water and snacks for the float (No Glass and No Styrofoam) 4. Make sure to show up on time. If you are 30 or more minutes late and do not contact us, you may be charged $20 an hour until you arrive. If you call us before your reservation time we are usually able to waive this fee. Meeting location is at mile marker 19 on Canyon Road between Ellensburg and Yakima. The current meeting location does not have an address. Please follow the directions below. Directions from I-90 (Heading either East or West): 1. Take exit 109, Ellensburg/Canyon road off of I-90 2. Turn left at the stop. 3. Continue on this road (Canyon Rd) for 9 miles 4. Look for mile marker 19 5. There is a wide section off the road with limited parking, park here and look for a Rill Adventures Employee. 6. Once you check in with a Rill Adventures employee you will follow the shuttle bus to Big Pines and get shuttled back mile marker 19.** **Big Pines Parking: You will need to pay $5 per car to park at Big Pines. Please bring cash with you for the parking fee. Every reservation comes with paddles and personal floatation devices (PFDs). Each person will be fitted with a PFD by a Rill Adventures staff member.