Larval Stages of the Crinoid-Associated Squat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

A New Classification of the Chirostyloidea (Crustacea: Decapoda: Anomura)
Zootaxa 2687: 56–64 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new classification of the Chirostyloidea (Crustacea: Decapoda: Anomura) KAREEN E. SCHNABEL1 & SHANE T. AHYONG2 1National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington, New Zealand. E-mail: [email protected] 2Australian Museum, 6 College Street, Sydney, NSW 2010 Australia. E-mail: [email protected] Abstract The high level classification of the Chirostyloidea Ortmann, 1892, is reviewed. Eumunididae Milne-Edwards & Bouvier, 1900, is resurrected for two genera formerly placed in the Chirostylidae Ortmann, 1892, Eumunida Smith, 1883, and Pseudomunida Haig, 1979, based on shared characteristics such as the dorsal carapace striation, presence of supraocular spines of the rostrum, dentition of the mandible, presence of an epipod and an annulated exopod flagellum of maxilliped 1. Three families are now included in the Chirostyloidea: Chirostylidae, Eumunididae and Kiwaidae. Diagnoses are provided for each family as well as a key to the families. The fossil record of the Chirostyloidea is discussed, with putative records of Eumunida in the fossil record referred to the galatheid genus Sadayoshia Baba, 1969. Key words: Galatheoidea, Chirostylidae, Eumunididae, Kiwaidae, adult somatic morphology, larval morphology, fossil record Introduction Recent focus on the phylogeny of Anomura has generated significant molecular phylogenetic information that has challenged the traditional understanding of the marine squat lobsters and porcelain crabs, the Galatheoidea, which comprised the Chirostylidae Ortmann, 1892, Galatheidae Samouelle, 1819, Porcellanidae Haworth, 1825, and Kiwaidae Macpherson, Jones & Segonzac, 2005 (e.g., Ahyong et al. -
A New Species of Squat Lobster of the Genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea
ZooKeys 935: 25–35 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.935.51931 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea Paula C. Rodríguez-Flores1,2, Enrique Macpherson1, Annie Machordom2 1 Centre d’Estudis Avançats de Blanes (CEAB-CSIC), C. acc. Cala Sant Francesc 14 17300 Blanes, Girona, Spain 2 Museo Nacional de Ciencias Naturales (MNCN-CSIC), José Gutiérrez Abascal, 2, 28006 Madrid, Spain Corresponding author: Paula C. Rodríguez-Flores ([email protected]) Academic editor: I.S. Wehrtmann | Received 10 March 2020 | Accepted 2 April 2020 | Published 21 May 2020 http://zoobank.org/E2D29655-B671-4A4C-BCDA-9A8D6063D71D Citation: Rodríguez-Flores PC, Macpherson E, Machordom A (2020) A new species of squat lobster of the genus Hendersonida (Crustacea, Decapoda, Munididae) from Papua New Guinea. ZooKeys 935: 25–35. https://doi. org/10.3897/zookeys.935.51931 Abstract Hendersonida parvirostris sp. nov. is described from Papua New Guinea. The new species can be distin- guished from the only other species of the genus, H. granulata (Henderson, 1885), by the fewer spines on the dorsal carapace surface, the shape of the rostrum and supraocular spines, the antennal peduncles, and the length of the walking legs. Pairwise genetic distances estimated using the 16S rRNA and COI DNA gene fragments indicated high levels of sequence divergence between the new species and H. granulata. Phylogenetic analyses, however, recovered both species as sister species, supporting monophyly of the genus. Keywords Anomura, mitochondrial genes, morphology, West Pacific Introduction Squat lobsters of the family Munididae Ahyong, Baba, Macpherson & Poore, 2010 are recognised by the trispinose or trilobate front, usually composed of a slender rostrum flanked by supraorbital spines (Ahyong et al. -

Anomura (Crustacea Decapoda) from the Mayotte Region, Western Indian Ocean
ATOLL RESEARCH BULLETIN NO. 593 ANOMURA (CRUSTACEA DECAPODA) FROM THE MAYOTTE REGION, WESTERN INDIAN OCEAN Joseph Poupin, Jean-Marie Bouchard, Vincent Dinhut, Régis Cleva, and Jacques Dumas ANOMURA (CRUSTACEA DECAPODA) FROM THE MAYOTTE REGION, WESTERN INDIAN OCEAN Joseph Poupin, Jean-Marie Bouchard, Vincent Dinhut, Régis Cleva and Jacques Dumas Atoll Research Bulletin No. 593 23 October 2013 All statements made in papers published in the Atoll Research Bulletin are the sole responsibility of the authors and do not necessarily represent the views of the Smithsonian Institution or of the editors of the Bulletin. Articles submitted for publication in the Atoll Research Bulletin should be original papers and must be made available by authors for open access publication. Manuscripts should be consistent with the “Author Formatting Guidelines for Publication in the Atoll Research Bulletin.” All submissions to the Bulletin are peer reviewed and, after revision, are evaluated prior to acceptance and publication through the publisher’s open access portal, Open SI (http://opensi.si.edu). Published by SMITHSONIAN INSTITUTION SCHOLARLY PRESS P.O. Box 37012, MRC 957 Washington, D.C. 20013-7012 www.scholarlypress.si.edu The rights to all text and images in this publication are owned either by the contributing authors or third parties. Fair use of materials is permitted for personal, educational, or noncommercial purposes. Users must cite author and source of content, must not alter or modify the content, and must comply with all other terms or restrictions that may be applicable. Users are responsible for securing permission from a rights holder for any other use. ISSN: 0077-5630 (online) i CONTENT CONTENT ............................................................................................................................. -

Nuevos Táxones Animales Descritos En La Península Ibérica Y Macaronesia Desde 1994 (XIII)
15. Especies_nuevas_2010 27/12/10 13:18 Página 313 CORE Graellsia,Metadata, 66(2): citation 313-344 and similar papers at core.ac.uk julio-diciembre 2010 Provided by MUCC (Crossref) ISSN: 0367-5041 doi:10.3989/graellsia.2010.v66.028 NOTICIA DE NUEVOS TÁXONES PARA LA CIENCIA EN EL ÁMBITO ÍBERO-BALEAR Y MACARONÉSICO Nuevos táxones animales descritos en la península PORIFERA Ibérica y Macaronesia desde 1994 (3ª parte) Dercitus (Dercitus) bucklandi lusitanicus Van Soest, Beglinger y De Voogd, 2010 Familia Pachastrellidae J. FERNÁNDEZ Museo Nacional de Ciencias Naturales, C.S.I.C. LOCALIDAD TIPO: Gettysburg Peak, Gorringe Bank, Portugal, océano José Gutiérrez Abascal, 2. 28006. Madrid. Atlántico, 31-38 m de profundidad. E-mail: [email protected] MATERIAL TIPO: holotipo (ZMA Por. 21810) en el National Centre for Biodiversity (antiguo Zoological Museum of the University of Amsterdam). DISTRIBUCIÓN: océano Atlántico (costas de Portugal y norte de España) y mar de Alborán. REFERENCIA: Van Soest, R.W.M., Beglinger, E.J. y De Voogd, N.J., De nuevo frente a otro capítulo de este trabajo. 2010. Skeletons in confusion: a review of astrophorid sponges Las consideraciones generales son las habituales y with (dicho–)calthrops as structural megascleres (Porifera, Demospongiae, Astrophorida). ZooKeys, 68: 1-88. no las repetiremos. NOTA: urn:lsid:zoobank.org:act:9152BD64-067E-4F21-AF03-6B84E6 Queremos expresar nuestro reconocimiento a 71E2A6 todos aquellos investigadores que nos han enviado Dercitus (Stoeba) senegalensis Van Soest, Beglinger y De Voogd, 2010 generosamente sus estudios y, de manera muy espe- Familia Pachastrellidae cial, a las personas que nos porporcionan informa- LOCALIDAD TIPO: costas frente a Senegal, océano Atlántico. -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

Kiwa Tyleri, a New Species of Yeti Crab from the East Scotia Ridge, Antarctica
RESEARCH ARTICLE Adaptations to Hydrothermal Vent Life in Kiwa tyleri, a New Species of Yeti Crab from the East Scotia Ridge, Antarctica Sven Thatje1*, Leigh Marsh1, Christopher Nicolai Roterman2, Mark N. Mavrogordato3, Katrin Linse4 1 Ocean and Earth Science, University of Southampton, European Way, Southampton, SO14 3ZH, United Kingdom, 2 National Oceanography Centre, Southampton, European Way, Southampton, SO14 3ZH, United Kingdom, 3 Engineering Sciences, μ-VIS CT Imaging Centre, University of Southampton, Southampton, SO17 1BJ, United Kingdom, 4 British Antarctic Survey, High Cross Madingley Road, CB3 0ET, Cambridge, United Kingdom a11111 * [email protected] Abstract Hydrothermal vents in the Southern Ocean are the physiologically most isolated chemosyn- OPEN ACCESS thetic environments known. Here, we describe Kiwa tyleri sp. nov., the first species of yeti Citation: Thatje S, Marsh L, Roterman CN, crab known from the Southern Ocean. Kiwa tyleri belongs to the family Kiwaidae and is the Mavrogordato MN, Linse K (2015) Adaptations to visually dominant macrofauna of two known vent sites situated on the northern and southern Hydrothermal Vent Life in Kiwa tyleri, a New Species segments of the East Scotia Ridge (ESR). The species is known to depend on primary pro- of Yeti Crab from the East Scotia Ridge, Antarctica. ductivity by chemosynthetic bacteria and resides at the warm-eurythermal vent environment PLoS ONE 10(6): e0127621. doi:10.1371/journal. pone.0127621 for most of its life; its short-range distribution away from vents (few metres) is physiologically constrained by the stable, cold waters of the surrounding Southern Ocean. Kiwa tylerihas Academic Editor: Steffen Kiel, Universität Göttingen, GERMANY been shown to present differential life history adaptations in response to this contrasting thermal environment. -

Anomura: Galatheidae) from the Dampier Archipelago, Western Australia
DOI: 10.18195/issn.0313-122x.73.2007.289-297 /-(ecords of the Western Australian ;\/useum Supplement No. 71: 2H9-297 (2007). Some new records of shallow-water galatheid crustaceans (Anomura: Galatheidae) from the Dampier Archipelago, Western Australia Enrique Macpherson Centro de Fstudios Avanzados de Blanes (CSIC) Cami ace. Cala San Francesc sin ]710() Blanes. Cirona. Spain. email: macphersolw1ceab.csic.es Abstract - A collection of galatheid crustaceans from the Dampier Archipelago, north-western Australia, is studied. Six species arc reported: AIJogalathea elegans (Adams and White, ]848), Ga/athea orientalis Stimpson, ]858, G. subsquamata Stimpson, 1858, Lauriea gardineri (Laurie, ](26), PhvIJadiorhynchus integrirostris (Dana, 1852) and Phylladiorhynchus muius sp. novo Ga/athea corallicola Haswell, 1888 is redescribed and its relationship with G. orientalis Stimpson, ]858 and G. coralliophilus Baba and Oh, 199() are discussed. INTRODUCTION SYSTEMATICS During recent expeditions to the Oampier Allogalathea elegans (Adams and White, 1848) Archipelago in 1998 and 1999, an interesting collection of shallow-water galatheids was Galathea elegans Adams and White, 1848: pI. 12, collected. The shallow-water galatheid fauna in this fig. 7. area of the Indian Ocean is not very well known, Allogalathea elegans Baba, 1969: 6, fig. 1. 1979: although some species have been reported, e.g 654, fig. 3. 1988: 54. Haig, 1973: 275. - 1974: Allogalathea elegans (Adams and White, 1848), 447. - Tirmizi and ]aved, 1993: 27, figs 12, 13. Galathea aegyptiaca (Paulson, 1875), G. austraJiensis Stimpson, 1858, G. corrallicola Material examined Haswell, 1882, G. genkai Miyake and Baba, 1964, G. Western Australia, Dampier Archipelago. WAM magnifica Haswell, 1882, G. pubescens Stimpson, C 26709 (2 males, 3.1-5.2 mm), stn OA2/99/06 1858, G. -
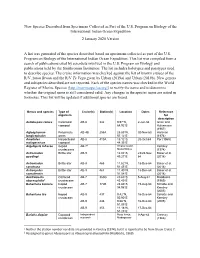
New Species Described from Specimens Collected As Part of the U.S
New Species Described from Specimens Collected as Part of the U.S. Program on Biology of the International Indian Ocean Expedition 2 January 2020 Version A list was generated of the species described based on specimens collected as part of the U.S. Program on Biology of the International Indian Ocean Expedition. This list was compiled from a search of publications cited by scientists involved in the U.S. Program on Biology and publications held by the Smithsonian Institution. The list includes holotypes and paratypes used to describe species. The cruise information was checked against the list of known cruises of the R/V Anton Bruun and the R/V Te Vega given by Urban (2019a) and Urban (2019b). New genera and subspecies described are not reported. Each of the species names was checked in the World Register of Marine Species (http://marinespecies.org/) to verify the name and to determine whether the original name is still considered valid. Any changes in the species name are noted in footnotes. This list will be updated if additional species are found. Genus and species Type of Cruise(s) Station(s) Location Dates Reference organism for description Aetideopsis retusa Calanaoid AB-6 342 9.97°S, 2-Jun-64 Grice and copepod 64.92°E Hulsemann (1967) Aglaophamus Polychaete AB-4B 255A 25.83°N, 30-Nov-63 Hartman longicephalus worm 57.12°E (1974) Ameliotes Harpacticoid AB-8 410A 15.12°S, 20-Oct-64 Por (1969) malagassicus copepod 44.35°E Argeiopsis inhacae isopod AB-71 Inhaca Island, Kensley crustaceans Mozambique (1974) Astrocladus Brittle star AB-9 12.83°S, 23/26-Nov- Baker et al. -

Allogalathea (Decapoda: Galatheidae): a Monospecific Genus of Squat Lobster?
Zoological Journal of the Linnean Society, 2011, 162, 245–270. With 7 figures Allogalathea (Decapoda: Galatheidae): a monospecific genus of squat lobster? PATRICIA CABEZAS1*, ENRIQUE MACPHERSON2 and ANNIE MACHORDOM1 1Museo Nacional de Ciencias Naturales (CSIC), José Gutiérrez Abascal 2, 28006 Madrid, Spain 2Centro de Estudios Avanzados de Blanes (CSIC), Carr. Acc. Cala Sant Francesc 14, 17300 Blanes, Girona, Spain Received 8 March 2010; revised 14 June 2010; accepted for publication 16 June 2010 The genus Allogalathea was established by Baba in 1969 to include the well-known species Galathea elegans. This species is widely distributed across the Indo-West Pacific Ocean, and is characterized by living in close association with crinoids, and by its conspicuous coloration. Although the genus is considered monospecific, different colour patterns and discrete morphological variations mainly associated with the rostrum and chelipeds have been reported. These differences could point to cryptic species, thereby questioning Allogalathea as a monotypic taxon. To address this issue, we sequenced the mitochondrial cytochrome oxidase I (COI; 658 bp) and 16S rRNA (882 bp) genes and the nuclear gene phosphoenolpyruvate carboxykinase (PEPCK; 598 bp) in numerous specimens from eight different localities, and also examined their morphological characters. DNA sequences were analysed using maximum-parsimony, maximum-likelihood, and Bayesian approaches of phylogenetic inference. The resulting trees were combined with morphological evidence to test species boundaries. Our molecular data revealed four deeply divergent clades, which can be distinguished by subtle morphological differences in the spinulation and length- : breadth ratio of the P1 carpus, spinulation of the walking legs, and shape of the rostrum. Our findings indicated that Allogalathea elegans is in fact a species complex comprising four different species, which, although genetically very distinct, are morphologically very similar. -

Associated with Gorgonian Corals from the Deep Waters Off Taiwan
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 120(2):167–174. 2007. AnewMunidopsis (Crustacea: Decapoda: Galatheidae) associated with gorgonian corals from the deep waters off Taiwan Chia-Wei Lin, Masayuki Osawa, and Tin-Yam Chan* (CWL, MO and TYC) Institute of Marine Biology, National Taiwan Ocean University, Keelung 202, Taiwan, R.O.C., email: [email protected]; (MO) Department of Marine and Environmental Sciences, University of the Ryukyus, 1 Senbaru, Nishihara-cho, Okinawa 903-0213, Japan Abstract.—Munidopsis sarissa, a new galatheid crustacean associated with gorgonian corals, is described from Taiwan at depths of about 1000 m. This new species is unique in the genus by having an extremely spinose carapace with elongated spearhead-like rostrum, fourth thoracic sternite much larger than following sternites, and very long and slender chelipeds possessing broad coxae which are clearly visible from the dorsal view of the animal. The galatheid genus Munidopsis White- species is deposited in the National aves, 1874 is predominantly deep-sea in Taiwan Ocean University, Keelung distribution and has a very high diversity, (NTOU). The postorbital carapace length with 147 species known in the Indo- (cl) was measured from the orbital margin Pacific and about 70 species in the to the posterior margin of the carapace Atlantic Ocean (Baba 1988, 2005; Mac- along the dorsal midline. The abbrevia- pherson & Segonzac 2005, Macpherson tions ‘‘CP’’ and ‘‘PCP’’ refer to the 2007). Species of the genus are often French beam trawl with the spans of 4.2 found in the catches of deep-sea benthic and 2.5 m, respectively. -

An Illustrated Key to the Malacostraca (Crustacea) of the Northern Arabian Sea. Part VI: Decapoda Anomura
An illustrated key to the Malacostraca (Crustacea) of the northern Arabian Sea. Part 6: Decapoda anomura Item Type article Authors Kazmi, Q.B.; Siddiqui, F.A. Download date 04/10/2021 12:44:02 Link to Item http://hdl.handle.net/1834/34318 Pakistan Journal of Marine Sciences, Vol. 15(1), 11-79, 2006. AN ILLUSTRATED KEY TO THE MALACOSTRACA (CRUSTACEA) OF THE NORTHERN ARABIAN SEA PART VI: DECAPODA ANOMURA Quddusi B. Kazmi and Feroz A. Siddiqui Marine Reference Collection and Resource Centre, University of Karachi, Karachi-75270, Pakistan. E-mails: [email protected] (QBK); safianadeem200 [email protected] .in (FAS). ABSTRACT: The key deals with the Decapoda, Anomura of the northern Arabian Sea, belonging to 3 superfamilies, 10 families, 32 genera and 104 species. With few exceptions, each species is accompanied by illustrations of taxonomic importance; its first reporter is referenced, supplemented by a subsequent record from the area. Necessary schematic diagrams explaining terminologies are also included. KEY WORDS: Malacostraca, Decapoda, Anomura, Arabian Sea - key. INTRODUCTION The Infraorder Anomura is well represented in Northern Arabian Sea (Paldstan) (see Tirmizi and Kazmi, 1993). Some important investigations and documentations on the diversity of anomurans belonging to families Hippidae, Albuneidae, Lithodidae, Coenobitidae, Paguridae, Parapaguridae, Diogenidae, Porcellanidae, Chirostylidae and Galatheidae are as follows: Alcock, 1905; Henderson, 1893; Miyake, 1953, 1978; Tirmizi, 1964, 1966; Lewinsohn, 1969; Mustaquim, 1972; Haig, 1966, 1974; Tirmizi and Siddiqui, 1981, 1982; Tirmizi, et al., 1982, 1989; Hogarth, 1988; Tirmizi and Javed, 1993; and Siddiqui and Kazmi, 2003, however these informations are scattered and fragmentary. In 1983 McLaughlin suppressed the old superfamily Coenobitoidea and combined it with the superfamily Paguroidea and placed all hermit crab families under the superfamily Paguroidea.