Iron Isotope Cosmochemistry Kun Wang Washington University in St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phobos, Deimos: Formation and Evolution Alex Soumbatov-Gur
Phobos, Deimos: Formation and Evolution Alex Soumbatov-Gur To cite this version: Alex Soumbatov-Gur. Phobos, Deimos: Formation and Evolution. [Research Report] Karpov institute of physical chemistry. 2019. hal-02147461 HAL Id: hal-02147461 https://hal.archives-ouvertes.fr/hal-02147461 Submitted on 4 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Phobos, Deimos: Formation and Evolution Alex Soumbatov-Gur The moons are confirmed to be ejected parts of Mars’ crust. After explosive throwing out as cone-like rocks they plastically evolved with density decays and materials transformations. Their expansion evolutions were accompanied by global ruptures and small scale rock ejections with concurrent crater formations. The scenario reconciles orbital and physical parameters of the moons. It coherently explains dozens of their properties including spectra, appearances, size differences, crater locations, fracture symmetries, orbits, evolution trends, geologic activity, Phobos’ grooves, mechanism of their origin, etc. The ejective approach is also discussed in the context of observational data on near-Earth asteroids, main belt asteroids Steins, Vesta, and Mars. The approach incorporates known fission mechanism of formation of miniature asteroids, logically accounts for its outliers, and naturally explains formations of small celestial bodies of various sizes. -

R-Process Elements from Magnetorotational Hypernovae
r-Process elements from magnetorotational hypernovae D. Yong1,2*, C. Kobayashi3,2, G. S. Da Costa1,2, M. S. Bessell1, A. Chiti4, A. Frebel4, K. Lind5, A. D. Mackey1,2, T. Nordlander1,2, M. Asplund6, A. R. Casey7,2, A. F. Marino8, S. J. Murphy9,1 & B. P. Schmidt1 1Research School of Astronomy & Astrophysics, Australian National University, Canberra, ACT 2611, Australia 2ARC Centre of Excellence for All Sky Astrophysics in 3 Dimensions (ASTRO 3D), Australia 3Centre for Astrophysics Research, Department of Physics, Astronomy and Mathematics, University of Hertfordshire, Hatfield, AL10 9AB, UK 4Department of Physics and Kavli Institute for Astrophysics and Space Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 5Department of Astronomy, Stockholm University, AlbaNova University Center, 106 91 Stockholm, Sweden 6Max Planck Institute for Astrophysics, Karl-Schwarzschild-Str. 1, D-85741 Garching, Germany 7School of Physics and Astronomy, Monash University, VIC 3800, Australia 8Istituto NaZionale di Astrofisica - Osservatorio Astronomico di Arcetri, Largo Enrico Fermi, 5, 50125, Firenze, Italy 9School of Science, The University of New South Wales, Canberra, ACT 2600, Australia Neutron-star mergers were recently confirmed as sites of rapid-neutron-capture (r-process) nucleosynthesis1–3. However, in Galactic chemical evolution models, neutron-star mergers alone cannot reproduce the observed element abundance patterns of extremely metal-poor stars, which indicates the existence of other sites of r-process nucleosynthesis4–6. These sites may be investigated by studying the element abundance patterns of chemically primitive stars in the halo of the Milky Way, because these objects retain the nucleosynthetic signatures of the earliest generation of stars7–13. -

(121514) 1999 UJ7: a Primitive, Slow-Rotating Martian Trojan G
A&A 618, A178 (2018) https://doi.org/10.1051/0004-6361/201732466 Astronomy & © ESO 2018 Astrophysics ? (121514) 1999 UJ7: A primitive, slow-rotating Martian Trojan G. Borisov1,2, A. A. Christou1, F. Colas3, S. Bagnulo1, A. Cellino4, and A. Dell’Oro5 1 Armagh Observatory and Planetarium, College Hill, Armagh BT61 9DG, Northern Ireland, UK e-mail: [email protected] 2 Institute of Astronomy and NAO, Bulgarian Academy of Sciences, 72, Tsarigradsko Chaussée Blvd., 1784 Sofia, Bulgaria 3 IMCCE, Observatoire de Paris, UPMC, CNRS UMR8028, 77 Av. Denfert-Rochereau, 75014 Paris, France 4 INAF – Osservatorio Astrofisico di Torino, via Osservatorio 20, 10025 Pino Torinese (TO), Italy 5 INAF – Osservatorio Astrofisico di Arcetri, Largo E. Fermi 5, 50125, Firenze, Italy Received 15 December 2017 / Accepted 7 August 2018 ABSTRACT Aims. The goal of this investigation is to determine the origin and surface composition of the asteroid (121514) 1999 UJ7, the only currently known L4 Martian Trojan asteroid. Methods. We have obtained visible reflectance spectra and photometry of 1999 UJ7 and compared the spectroscopic results with the spectra of a number of taxonomic classes and subclasses. A light curve was obtained and analysed to determine the asteroid spin state. Results. The visible spectrum of 1999 UJ7 exhibits a negative slope in the blue region and the presence of a wide and deep absorption feature centred around ∼0.65 µm. The overall morphology of the spectrum seems to suggest a C-complex taxonomy. The photometric behaviour is fairly complex. The light curve shows a primary period of 1.936 d, but this is derived using only a subset of the photometric data. -

Recent Observations of the Soils on the Moon
Lunar Soil for a Myriad of Purposes and Products: Science, Engineering, and Applications LawrenceLawrence A.A. TaylorTaylor PPlanetary Geosciences Institute University of Tennessee Objectives NEW LUNAR SAMPLES UNIQUE PROPERTIES OF LUNAR SOIL NEW LUNAR MINERALS UNIQUE ABILITIES OF MICROWAVE RADIATION MICROWAVE PROCESSING OF LUNAR SOIL PRODUCTS FOR “THE BUSH LUNAR BASE” Newly Recovered Planetary Materials Meteorites from Antarctica Japan and USA Meteorites from Hot Deserts French Entrepreneurs in the Sahara Russian Entrepreneurs in Oman Meteorites as samples of : as well as Mars & Asteroidal Matter SEARCHING FOR METEORITES IN THE OMAN DESERT When viewed with binoculars, the Black Spot in the sand is: a) a ‘coke can’ [alcohol is verboten!]; b) evidence that a camel has stopped there recently; or c) a dark-colored rock [fusion crust on a meteorite]. Meteorite? LunarLunar MeteoritesMeteorites fromfrom OmanOman Dhofar 287 Dhofar 025 (Impact-Melt Breccia) (Basalt + Breccia) Regolith Breccia Dhofar 301 Dhofar 302 Dhofar 303 New Lunar Rocks and Minerals: Farside Samples of the Moon South Pole / Amundsen Site Volcanic Glass Impact-Glass Lunar Mare Soil Bead Bead Agglutinate Rock Chips Impact Glass 1 mm Plagioclase Our Unhappy Moon MicrometeoriteMicrometeorite ImpactsImpacts onon LunarLunar GlassGlass BeadBead 5 µm Impact Craters LunarLunar SoilSoil FormationFormation Comminution, Agglutination, & Vapor Deposition TheThe onlyonly WeatheringWeathering andand EErosionalrosional agentagent onon thethe MoonMoon isis MeteoriteMeteorite andand MicrometeoriteMicrometeorite -

Moon Minerals a Visual Guide
Moon Minerals a visual guide A.G. Tindle and M. Anand Preliminaries Section 1 Preface Virtual microscope work at the Open University began in 1993 meteorites, Martian meteorites and most recently over 500 virtual and has culminated in the on-line collection of over 1000 microscopes of Apollo samples. samples available via the virtual microscope website (here). Early days were spent using LEGO robots to automate a rotating microscope stage thanks to the efforts of our colleague Peter Whalley (now deceased). This automation speeded up image capture and allowed us to take the thousands of photographs needed to make sizeable (Earth-based) virtual microscope collections. Virtual microscope methods are ideal for bringing rare and often unique samples to a wide audience so we were not surprised when 10 years ago we were approached by the UK Science and Technology Facilities Council who asked us to prepare a virtual collection of the 12 Moon rocks they loaned out to schools and universities. This would turn out to be one of many collections built using extra-terrestrial material. The major part of our extra-terrestrial work is web-based and we The authors - Mahesh Anand (left) and Andy Tindle (middle) with colleague have build collections of Europlanet meteorites, UK and Irish Peter Whalley (right). Thank you Peter for your pioneering contribution to the Virtual Microscope project. We could not have produced this book without your earlier efforts. 2 Moon Minerals is our latest output. We see it as a companion volume to Moon Rocks. Members of staff -

Concrete Analysis by Neutron-Capture Gamma Rays Using Californium 252
CONCRETE ANALYSIS BY NEUTRON-CAPTURE GAMMA RAYS USING CALIFORNIUM 252 Dick Duffey, College of Engineering, University of Maryland; Peter F. Wiggins, Naval Systems Engineering Department, U.S. Naval Academy; Frank E. Senftle, U.S. Geological Survey; and A. A. El Kady, United Arab Republic Atomic Energy Establishment, Cairo The feasibility of analyzing concrete and cement by a measurement of the neutron-capture or prompt gamma rays was investigated; a 100-ug californium-252 source was used to supply the neutrons. A lithium drifted germanium crystal detected the capture gamma rays emitted. The capture gamma rays from cement, sand, and 3 coarse aggregates-quartzite gravel, limestone, and diabase--:were studied. Concrete blocks made from these materials were then tested. The capture gamma ray response of the calcium, silicon, and iron in the concrete blocks was in accord with the elements identified in the mix materials. The principal spectral lines used were the 6.42 MeV line of calcium, the 4.93 MeV line of silicon, and the doublet of iron at about 7 .64 MeV. The aluminum line at 7. 72 MeV was ob served in some cases but at a lower intensity with the limited electronic equipment available. This nuclear spectroscopic technique offers a possi ble method of determining the components of sizable concrete samples in a nondestructive, in situ manner. In addition, the neutron-capture gamma ray technique might find application in control of the concrete and cement processes and furnish needed information on production operations and inventories. • FROM THE point of view of the geochemical analyst, concrete may be considered as rock relocated and reformed at the convenience of the engineer. -

Nuclear Astrophysics: the Unfinished Quest for the Origin of the Elements
Nuclear astrophysics: the unfinished quest for the origin of the elements Jordi Jos´e Departament de F´ısica i Enginyeria Nuclear, EUETIB, Universitat Polit`ecnica de Catalunya, E-08036 Barcelona, Spain; Institut d’Estudis Espacials de Catalunya, E-08034 Barcelona, Spain E-mail: [email protected] Christian Iliadis Department of Physics & Astronomy, University of North Carolina, Chapel Hill, North Carolina, 27599, USA; Triangle Universities Nuclear Laboratory, Durham, North Carolina 27708, USA E-mail: [email protected] Abstract. Half a century has passed since the foundation of nuclear astrophysics. Since then, this discipline has reached its maturity. Today, nuclear astrophysics constitutes a multidisciplinary crucible of knowledge that combines the achievements in theoretical astrophysics, observational astronomy, cosmochemistry and nuclear physics. New tools and developments have revolutionized our understanding of the origin of the elements: supercomputers have provided astrophysicists with the required computational capabilities to study the evolution of stars in a multidimensional framework; the emergence of high-energy astrophysics with space-borne observatories has opened new windows to observe the Universe, from a novel panchromatic perspective; cosmochemists have isolated tiny pieces of stardust embedded in primitive meteorites, giving clues on the processes operating in stars as well as on the way matter condenses to form solids; and nuclear physicists have measured reactions near stellar energies, through the combined efforts using stable and radioactive ion beam facilities. This review provides comprehensive insight into the nuclear history of the Universe arXiv:1107.2234v1 [astro-ph.SR] 12 Jul 2011 and related topics: starting from the Big Bang, when the ashes from the primordial explosion were transformed to hydrogen, helium, and few trace elements, to the rich variety of nucleosynthesis mechanisms and sites in the Universe. -

74 Dauphas Et Al EPSL 2014.Pdf
Earth and Planetary Science Letters 398 (2014) 127–140 Contents lists available at ScienceDirect Earth and Planetary Science Letters www.elsevier.com/locate/epsl Magma redox and structural controls on iron isotope variations in Earth’s mantle and crust ∗ N. Dauphas a, , M. Roskosz b, E.E. Alp c, D.R. Neuville d,M.Y.Huc,C.K.Sioa, F.L.H. Tissot a, J. Zhao c, L. Tissandier e,E.Médardf,C.Cordierb a Origins Laboratory, Department of the Geophysical Sciences and Enrico Fermi Institute, The University of Chicago, 5734 South Ellis Avenue, Chicago, IL 60637, USA b Unité Matériaux et Transformations, Université Lille 1, CNRS UMR 8207, 59655 Villeneuve d’Ascq, France c Advanced Photon Source, Argonne National Laboratory, 9700 South Cass Avenue, Argonne, IL 60439, USA d Géochimie et Cosmochimie, IPGP-CNRS, Sorbonne Paris Cité, 1 rue Jussieu, 75005 Paris, France e Centre de Recherches Pétrographiques et Géochimiques, CNRS-UPR 2300, 15 rue Notre-Dame des Pauvres, BP20, 54501 Vandoeuvre-lès-Nancy, France f Laboratoire Magmas et Volcans, Université Blaise Pascal, CNRS, IRD, 5 rue Kessler, 63038 Clermont-Ferrand, France article info abstract Article history: The heavy iron isotopic composition of Earth’s crust relative to chondrites has been explained by Received 13 July 2013 vaporization during the Moon-forming impact, equilibrium partitioning between metal and silicate at Received in revised form 15 April 2014 core–mantle-boundary conditions, or partial melting and magma differentiation. The latter view is Accepted 21 April 2014 supported by the observed difference in the iron isotopic compositions of MORBS and peridotites. -

Proposed Mission to Mars and His Trojan Asteroid Family
Geophysical Research Abstracts Vol. 21, EGU2019-13883, 2019 EGU General Assembly 2019 © Author(s) 2019. CC Attribution 4.0 license. Proposed mission to Mars and his Trojan Asteroid Family Kai Wickhusen (1), Jürgen Oberst (1,2), and Konrad Willner (1) (1) German Aerospace Center (DLR), Berlin, Germany ([email protected]), (2) Institute of Geodesy and Geoinformation Science, Technical University of Berlin, Germany In the context of ESA’s past call for medium class missions, we were investigating missions to Mars in combina- tion with a flyby at a Mars Trojan on the way to the planet. We set up a three-impulse (Earth departure, mid-course correction and Mars orbit insertion) model to determine physically possible trajectories and flyby scenarios op- timized in terms of required propellant and time (i.e. operation costs). We propose several scenarios in the time frame 2020-2050 including trajectories that involve more than one revolution about the sun. With the Earth and Mars ephemerides relatively fixed in space and time, the Trojan candidate has to be “at the right place at the right time” to minimize expensive spacecraft course adjustments. In principle, flybys are possible for any Trojan target – but we cut-off and present models having flyby delta-v < 1 km/s and transit time of < 5 years. We report on several scenarios, each including a flyby at one Trojan. Our results show that flybys of most of the Trojans are well conceivable. One good candidate for such a mission is Trojan 311999 (2007 NS2). With a launch in September 2028, the required additional delta-v will be less than 100 m/s, associated with a total transfer time (from Earth to Mars) of approximately 800 days. -

Alpha Process
Alpha process The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.[1] The triple-alpha process consumes only helium, and produces carbon. After enough carbon has accumulated, the reactions below take place, all consuming only helium and the product of the previous reaction. E is the energy produced by the reaction, released primarily as gamma rays (γ). It is a common misconception that the above sequence ends at (or , which is a decay product of [2]) because it is the most stable nuclide - i.e., it has the highest nuclear binding energy per nucleon, and production of heavier nuclei requires energy (is endothermic) instead of releasing it (exothermic). (Nickel-62) is actually the most stable nuclide.[3] However, the sequence ends at because conditions in the stellar interior cause the competition between photodisintegration and the alpha process to favor photodisintegration around iron,[2][4] leading to more being produced than . All these reactions have a very low rate at the temperatures and densities in stars and therefore do not contribute significantly to a star's energy production; with elements heavier than neon (atomic number > 10), they occur even less easily due to the increasing Coulomb barrier. Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of four, the mass of the helium nucleus (the alpha particle); these isotopes are known as alpha nuclides. Stable alpha elements are: C, O, Ne, Mg, Si, and S; Ar and Ca are observationally stable. -

In Iron Silicide
CALCIUM-ALUMINUM-RICH INCLUSIONS (CAIs) IN IRON SILICIDE (XIFENGITE, GUPEIITE, HAPKEITE) 76th Annual Meteoritical Society Meeting (2013) MATTER: EVIDENCE OF A COSMIC ORIGIN 5055.pdf Abstract The CAIs Mm- to cm-sized metallic particles in the subsoil of -- The iron silicides from the Chiemgau impact strewn field contain CAIs with M.A. Rappenglück the Alpine Foreland are composed of iron silicides minerals CaAl2O4, calcium monoaluminate, and Ca2Al2O5, dicalcium dialuminate. Institute for Interdisciplinary Studies, Fe3Si, mineral gupeiite, Fe5Si3, mineral xifengite, -- The monoclinic high-temperature (>1,500°C), low-pressure dimorph of CaAl2O4, Gilching, Germany, [email protected] Fe2Si, mineral hapkeite, FeSi, fersilicite, and FeSi2, mineral krotite, was first identified in a CAI from the CH chondrite NWA 470 [17] ferdisilicite, the minerals gupeiite, xifengite and and later reported [18, 19] to exist in a CAI in the carbonaceous chondrite meteorite F. Bauer 10 µm 2 µm NWA 1934. fersilicite being the main components. More peculiar Oxford Instruments GmbH Nano- Science, mineral components add to the matrix also hosting -- The orthorhombic Ca2Al2O5 dicalcium dialuminate high pressure phase with the Fig. 6. Zircon crystals obviously having impacted larger crystals of extremely pure titanium carbide, Fig. 5. Zircon crystals in iron silicide matrix. brownmillerite-type structure was established in 2000 [20] and has so far no natural Wiesbaden Germany, The white tips on the crystals have been shown a plastic or liquid iron silicide matrix that seems [email protected] mineral khamrabaevite, and silicon carbide, mineral to be uranium. to have been frozen during the disturbance. counterpart. Experimental data were 1,250°C and 2.5 GPa, and stability was reached moissanite. -
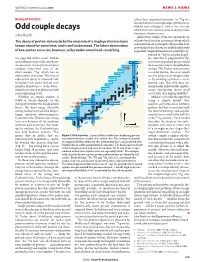
Odd Couple Decays Which Both One- and Two-Proton Decay Modes Juha Äystö Have Been Shown to Exist
19.1 N&V 273 MH 13/1/06 5:21 PM Page 279 NATURE|Vol 439|19 January 2006 NEWS & VIEWS NUCLEAR PHYSICS rather than sequential emission. As 94Ag was already known to undergo single-proton decay, Mukha and colleagues’ state is the first for Odd couple decays which both one- and two-proton decay modes Juha Äystö have been shown to exist. All previous studies of the two-proton decay The decay of proton-rich nuclei by the emission of a single proton has been of short-lived nuclear resonances from which known about for some time, and is well understood. The latest observation sequential decay is energetically possible have proved that these decays are indeed exclusively of two-proton emission, however, will provoke some head-scratching. sequential. Sequential emission is probably sup- pressed in 94Ag because the daugh- On page 298 of this issue1, Mukha ter state that is populated by the and colleagues report the simultane- Sn 50 first of two sequential decays would ous emission of two protons from a be an excited state of the palladium complex, long-lived state of the 94Ag isotope 93Pd. Rather than emitting silver isotope 94Ag, which has an a second proton, this state would odd number of protons. This type of tend to decay electromagnetically radioactive decay is expected only — by emitting a photon — to its for proton-rich nuclei with an even ground state. But why does the number of protons — so the obser- energetically unfavourable simulta- vation leaves nuclear physicists with neous two-proton decay itself some explaining to do.