Air Pollution, Heavy Metals, Bioindicator, Feces, Wild Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Indian Wolf (Canis Lupus Pallipes): II Edition
NATIONALNATIONAL STUDBOOKSTUDBOOK OF INDIAN WOLF (CANIS LUPUS PALLIPES) – II EDITION Indian Wolf (Canis lupusOK pallipes): II Edition NATIONAL STUDBOOK OF INDIAN WOLF (CANIS LUPUS PALLIPES) – II EDITION NATIONAL STUDBOOK OF INDIAN WOLF (CANIS LUPUS PALLIPES) – II EDITION National Studbook Indian Wolf (Canis lupuspallipes) II Edition Part of the Central Zoo Authority sponsored project titled “Development and Maintenance of Studbooks for Selected Endangered Species in Indian Zoos” awarded to the Wildlife Institute of India vide sanction order: Central Zoo Authority letter no. 9-2/2012-CZA(NA)/418 dated 7th March 2012 PROJECT TEAM Dr. Parag Nigam Principal Investigator Dr. Anupam Srivastav Project Consultant Ms. Neema Sangmo Lama Research Assistant Photo Credits: © Indrajit Ghorpade Copyright © WII, Dehradun, and CZA, New Delhi, 2018 __________________________________________________________________________________ This report may be quoted freely but the source must be acknowledged and cited as: Wildlife Institute of India (2018). National Studbook of Indian Wolf (Canis lupus pallipes) II Edition, Wildlife Institute of India, Dehradun and Central Zoo Authority, New Delhi. TR.No. 2018/30. Pages 90. NATIONAL STUDBOOK OF INDIAN WOLF (CANIS LUPUS PALLIPES) – II EDITION NATIONAL STUDBOOK OF INDIAN WOLF (CANIS LUPUS PALLIPES) – II EDITION FOREWORD Habitat loss, fragmentation and degradation, retaliatory killing coupled with disease threats have rendered Indian wolves prone to extinction. Despite protection measures in recent times free ranging populations continue to show declines; therefore, maintenance of viable ex-situ populations for ensuring their long-term persistence remains imperative. Scientific management that ensures their long-term genetic viability and demographic stability can ensure effective ex-situ conservation of Indian wolves. Pedigree information contained in studbooks forms the basis for this effort. -

Characterising the Harmonic Vocal Repertoire of the Indian Wolf (Canis Lupus Pallipes)
RESEARCH ARTICLE Characterising the harmonic vocal repertoire of the Indian wolf (Canis lupus pallipes) 1 2 1 Sougata SadhukhanID , Lauren HennellyID , Bilal HabibID * 1 Department of Animal Ecology and Conservation Biology, Wildlife Institute of India, Dehradun, India;, 2 Mammalian Ecology and Conservation Unit, Veterinary Genetics Laboratory, University of California Davis, Davis, California, United States of America * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Vocal communication in social animals plays a crucial role in mate choice, maintaining social structure, and foraging strategy. The Indian grey wolf, among the least studied subspecies, is a social carnivore that lives in groups called packs and has many types of vocal communi- cation. In this study, we characterise harmonic vocalisation types of the Indian wolf using howl survey responses and opportunistic recordings from captive and nine packs (each OPEN ACCESS pack contains 2±9 individuals) of free-ranging Indian wolves. Using principal component Citation: Sadhukhan S, Hennelly L, Habib B (2019) analysis, hierarchical clustering, and discriminant function analysis, we found four distinct Characterising the harmonic vocal repertoire of the Indian wolf (Canis lupus pallipes). PLoS ONE 14 vocalisations using 270 recorded vocalisations (Average Silhouette width Si = 0.598) which (10): e0216186. https://doi.org/10.1371/journal. include howls and howl-barks (N = 238), whimper (N = 2), social squeak (N = 28), and whine pone.0216186 (N = 2). Although having a smaller body size compared to other wolf subspecies, Indian wolf Editor: Govindhaswamy Umapathy, Centre for howls have an average mean fundamental frequency of 422 Hz (±126), which is similar to Cellular and Molecular Biology, INDIA other wolf subspecies. -

AR Nahargarh 1819.Pdf
CONTENTS S. No. Section Page Number 1. Report of the Officer-in-charge 1 2. History of the Zoo 1-2 3. Vision 2 4. Mission 2 5. Objective 2 6. About us 3-4 7. Organizational Chart 4 8. Human Resources 5 9. Capacity Building of the zoo personnel 5 10. Zoo Advisory Committee 5 11. Health Advisory Committee 5 12. Statement of income and expenditure of the Zoo 6-7 13. Daily feed Schedule of animals 7-9 14. Vaccination Schedule of animals 9-10 15. De-worming Schedule of animals 10 16. Disinfection Schedule 10 17. Health Check-up of employees for zoonotic diseases 10 S. No. Section Page Number 18. Development Works carried out in the zoo during the year 11 19. Education and Awareness programmes during the year 11-13 20. Important Events and happenings in the zoo 13-14 21. Seasonal special arrangements for upkeep of animals 14 22. Research Work carried out and publications 14 23. Conservation Breeding Programme of the Zoo 14-15 24. Animal acquisition / transfer / exchange during the year 15 Rescue and Rehabilitation of the wild animals carried out by 25. 15-19 the zoo 26. Annual Inventory of animals 20-23 27. Mortality of animals. 24-25 Status of the Compliance with conditions stipulated by the 28. 25-34 Central Zoo Authority 29. List of free living wild animals within the zoo premises 34-38 30. TREE TRANSPLANTATION 39-40 1. Report Of The Officer-In-Charge Shri Sudarshan Sharma Deputy Conservator of Forests (Wildlife), Zoo, Jaipur Nahargarh Zoological Park is one of the main tourist attraction in Jaipur, the second world heritage city of India. -

Characterising the Vocal Repertoire of the Indian Wolf (Canis Lupus Pallipes)
bioRxiv preprint doi: https://doi.org/10.1101/612507; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Characterising the vocal repertoire of the Indian wolf 2 (Canis lupus pallipes) 3 Sougata Sadhukhana, Lauren Hennellyb and Bilal Habiba* 4 a Department of Animal Ecology and Conservation Biology, Wildlife Institute of India, 5 Dehradun, India; 6 b Mammalian Ecology and Conservation Unit, Veterinary Genetics Laboratory, 7 University of California, Davis, USA 8 * Corresponding Author 9 Dr Bilal Habib, Scientists-E, Department of Animal Ecology and Conservation Biology, 10 Wildlife Institute of India, Dehradun-248001, India, Email ID- [email protected] 11 1 bioRxiv preprint doi: https://doi.org/10.1101/612507; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 13 Characterising the vocal repertoire of the Indian wolf 14 (Canis lupus pallipes) 15 Vocal communication in social animals plays a crucial role in mate choice, 16 maintaining social structure, and foraging strategy. The Indian grey wolf, among 17 the less studied subspecies, is a social carnivore that lives in groups called packs 18 and has many types of vocal communication. In this study, we characterise 19 harmonic vocalisation types in the Indian wolf using howl survey responses and 20 opportunistic recordings from captive and nine packs (each pack contains 2-9 21 individuals) of free-ranging Indian wolves. -

The Behaviour of Indian Gray Wolf (Canius Lupus Pallipes) in Captivity at Sakkarbaug Zoo, Junagadh, Gujarat, India
International Journal of Environment, Ecology, Family and Urban Studies (IJEEFUS) ISSN (P): 2250-0065; ISSN (E): 2321-0109 Vol. 9, Issue 2, Apr 2019, 55-68 © TJPRC Pvt. Ltd. THE BEHAVIOUR OF INDIAN GRAY WOLF ( Canius lupus pallipes ) IN CAPTIVITY AT SAKKARBAUG ZOO JUNAGADH, GUJARAT, INDIA MAITRY S. DAVE & JATIN V. RAVAL Zoology Lab, Department of Life Sciences, Bhakta Kavi Narsinh Mehta University, Junagadh, Gujarat, India ABSTRACT The present study was carried out on the behaviour of Indian gray wolf (Canius lupus pallipes) in captivity at Sakkarbaug Zoo, Junagadh, Gujarat, India. The Indian gray wolf is critically endangered species and fall into schedule- 1. Total ten wolves of 3-4 years old were observed. Ninety minutes observation was taken every week at morning time. Walking, resting, facial, resting alert, playing, fighting, standing, eating, etc. behaviours were studied. The observations of various behaviour activities of wolves we rerecorded from July 2018 to January 2019. Two different types of behaviours like active behaviour and inactive behaviours observed with two different patterns as 1) Wolf was solo in captivity and 2) Wolf pack in captivity. The time slice method was used. The wolves’ activity was registered in each ten Article Original minutes, nine times in one and half hour at morning. Twelve types of behavioural activities were recorded in the captive wolves. They were displaying different types of activities. Peaceful sharing of food with one another without competition was found in wolves. The newly born pups and mother were kept in separate cage from other captive wolves’ cage for their better survival and growth. -

Central Zoo Authority -- List of Rec Zoos.Pmd
Central Zoo Authority and Indian Zoos: A Current Overview Sally Walker Central Zoo Authority (CZA) (estab. Feb. 1992) is now a dozen years old. The Authority exists at the pleasure of the Central Government, which has reconstituted it four times and will undoubtedly do so again in the immediate future. CZA was set up to tackle many problems, among the most important, the rampant proliferation of zoos without adequate plan or principles, or even financial backing. Many of the then ~ 350 zoos had been set up for political reasons, to satisfy the whims of a Minister or other official and left to deteriorate after the founding function. It is very easy to open a zoo compared to closing one but this task -- closing zoos that could not meet a minimum standard -- was given to the fledgling Authority This was, part of a process, also led by CZA, to provide oversight for zoo management and, after evaluation, to give technical and monetary support and time, so that the zoos could improve. In this process, which is well established today, if the zoos improve sufficiently, they are granted recognition for three years. If they do not improve within a specified time frame they are deemed "derecognised" and must be closed by their state. Only recognised zoo can operate legally in the country. As of this year there are 164 recognised zoos in the country and 90 recently derecognised zoos. Since a few years after the inception of CZA, many other zoos have been derecognised. This article is an update and overview of some of the last year's activities of Central Zoo Authority. -

CBSG Annual Meeting 30 October–2 November 2014 ♦ New Delhi, India
Working Group Regional Network Meeting Participant Summaries Page 4 Updates Page 13 List Page 17 CBSG Annual Meeting 30 October–2 November 2014 ♦ New Delhi, India In October, the CBSG headquarters staff and regional network teams traveled to New Delhi, India for the 2014 CBSG Annual Meeting. One hundred and twenty people from 24 countries gathered to discuss the application of the One Plan approach in India and beyond. Our hosts, the Central Zoo Authority, India (CZA) outdid themselves with detailed planning and smooth implementation of the entire event, from the lovely opening reception to the closing dinner under the stars. We are grateful to B.S. Bonal, Brij Gupta, Sally Walker, Sanjay Molur, and the CBSG South Asia staff for making this a meeting to remember. The three days contained many fine examples of conservation in action around the world, including generation of population modeling tool developers; conservation of gyps vultures in India, linking in situ– continue the evolution of VORTEX; and drive ongoing ex situ efforts in greater one-horned rhino conservation, innovation of metamodeling tools for comprehensive, and the focus on integrated conservation in Indian zoos. integrated assessments of the many threats affecting Click here to read plenary presentation abstracts. wildlife. The participants met in working groups to discuss some • IUCN Guidelines of the key issues facing our community and the wildlife Three working groups received overviews on the we are dedicated to protecting. Examples include: application of some new or recently revised IUCN guidelines for reintroduction, ex situ management, • IUCN Asian Species Action Partnership (ASAP) and wildlife health. -

February 2016 FIELD FORESTER | February 2016
FIELD FORESTER V OICES FROM THE FIELD volume 1 ● issue 4 FEBRUARY 2016 FIELD FORESTER | February 2016 Patron: Dr S S Negi Director General of Forests Ministry of Environment, Forests and Climate Change Government of India Editorial Advisory Board Chief Advisor: Member Secretary: Dr Anil Kumar Shri M P Singh Additional Director General Director Forest Education of Forests (FC) Directorate of Forest Education Ministry of Environment, Ministry of Environment, Forests and Climate Change Forests & Climate Change Government of India Government of India Advisor: Advisor: Dr Suneesh Buxy Shri Deepak Mishra Deputy Inspector General of Additional Professor Forests (RT) Indira Gandhi National Forest Ministry of Environment, Academy Forests and Climate Change Dehradun Government of India Members: ● Principal, Central Academy for State Forest Service, Dehradun ● Principal, Central Academy for State Forest Service, Coimbatore ● Principal, Central Academy for State Forest Service, Burnihat ● Principal, Eastern Forest Rangers College, Kurseong ● Director, Telangana State Forest Academy, Dulapally, Hyderabad, Telangana ● Director, Uttarakhand Forestry Training Academy, Haldwani, Uttarakhand ● Director, Forest Training Institute & Rangers College, Sunder Nagar, Himachal Pradesh ● Director, Tamil Nadu State Forest Academy, Coimbatore, Tamil Nadu ● Director, Forest Training Institute, Gungargatti, Dharwad, Karnataka ● Director, Kundal Academy of Development, Administration & Management, Kundal, Maharashtra CONTENTS ii FIELD FORESTER | February 2016 Editorial -

Answered On:19.03.2001 Zoological Parks Rasa Singh Rawat
GOVERNMENT OF INDIA ENVIRONMENT AND FORESTS LOK SABHA UNSTARRED QUESTION NO:3229 ANSWERED ON:19.03.2001 ZOOLOGICAL PARKS RASA SINGH RAWAT Will the Minister of ENVIRONMENT AND FORESTS be pleased to state: (a) the number of zoological parks functioning in the country at present and the number of species kept therein, State-wise; (b) whether there has been lack of fund provision for the zoological parks; (c) if so, the action taken by the Government to remedy the situation? Answer MINISTER OF ENVIRONMENT & FORESTS (SHRI T.R. BAALU) (a) There are 182 recognized zoological parks in the country at present. Total number of species kept in the major zoos (Large, Medium and Small) is at Annexure- I (b)&(c) Due to financial crunch the State Governments are not in a position to provide adequate funds for maintenance of zoos. However, Central Zoo Authority has been providing financial assistance for different zoos in the country for development of infrastructure, better upkeep and health care of the animals. Annexure- I ANNEXURE REFERRED TO IN REPLY TO PART (Oa)F LOK SABHA UNSTARRED QUESTION NO. 3229 FOR 19.3.2001 REGARDING ZOOLOGICAL PARKS No of Species held in Major Zoos Name of State Name of the Zoo Location Total No. of Species Total No. of animals Andhra Pradesh Indira Gandhi Zoological Park, 95 927 Vishakapatnam Andhra Pradesh Nehru Zoological Park Hyderabad 170 1586 Andhra Pradesh Sri Venkateswara Zoo, Tirupati 57 676 Arunachal Prad Zoological Park, Itanagar 35 95 Assam Assam State Zoo cum Botanical 100 654 Garden, Guwahati Bihar Sanjay Gandhi -
Environment and Forests on Environment: Rajya Sabha 2012-13 (Winter Session) – PART-III
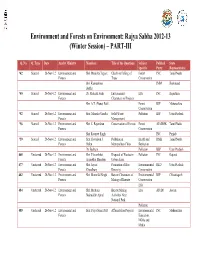
Environment and Forests on Environment: Rajya Sabha 2012-13 (Winter Session) – PART-III Q. No. Q. Type Date Ans by Ministry Members Title of the Questions Subject Political State Specific Party Representative *42 Starred 26-Nov-12 Environment and Shri Manicka Tagore Check on Felling of Forest INC Tamil Nadu Forests Trees Conservation Shri Kameshwar JMM Jharkhand Baitha *45 Starred 26-Nov-12 Environment and Dr. Mahesh Joshi Environment EIA INC Rajasthan Forests Clearances to Projects Shri A.T. (Nana) Patil Forest BJP Maharashtra Conservation *52 Starred 26-Nov-12 Environment and Smt. Maneka Gandhi Solid Waste Pollution BJP Uttar Pradesh Forests Management *56 Starred 26-Nov-12 Environment and Shri C. Rajendran Conservation of Forests Forest AIADMK Tamil Nadu Forests Conservation Shri Ravneet Singh INC Punjab *59 Starred 26-Nov-12 Environment and Smt. Davidson J. Pollution in Health and DMK Tamil Nadu Forests Helen Metropolitan Cities Sanitation Dr. Baliram Pollution BSP Uttar Pradesh 465 Unstarred 26-Nov-12 Environment and Shri Vikrambhai Disposal of Wastes in Pollution INC Gujarat Forests Arjanbhai Maadam Urban Areas 477 Unstarred 26-Nov-12 Environment and Shri Jayant Promotion of Bio- Environmental RLD Uttar Pradesh Forests Chaudhary Diversity Conservation 482 Unstarred 26-Nov-12 Environment and Shri Murarilal Singh Ban on Clearances of Environmental BJP Chhattisgarh Forests Mining of Bauxite Conservation EIA 484 Unstarred 26-Nov-12 Environment and Shri Maulana Ban on Mining EIA AIUDF Assam Forests Badruddin Ajmal Activities Near National Park Pollution 489 Unstarred 26-Nov-12 Environment and Smt. Priya Sunil Dutt Afforestation Projects Environmental INC Maharashtra Forests Education, NGOs and Media Forest Conservation 490 Unstarred 26-Nov-12 Environment and Shri N. -

Government of India Ministry of Environment, Forest and Climate Change
GOVERNMENT OF INDIA MINISTRY OF ENVIRONMENT, FOREST AND CLIMATE CHANGE LOK SABHA UNSTARRED QUESTION No. 2726 TO BE ANSWERED ON 06.03.2020. Animals in Zoo 2726. SHRIMATI PRATIMA MONDAL: Will the Minister of ENVIRONMENT, FOREST AND CLIMATE CHANGE be pleased to state: (a) the State/UT-wise list of zoos in the country along with the details of species of varied animals and their population; and (b) the steps taken by the Government to ensure proper care and empathetic treatment of these animals? ANSWER MINISTER OF STATE IN THE MINISTRY OF ENVIRONMENT, FOREST AND CLIMATE CHANGE (SHRI BABUL SUPRIYO) (a) The State/Union Territory-wise list of recognized Zoos in the country is at Annexure-1. The details of animals and their populations in Indian zoos is at Annexure-2. (b) The Central Zoo Authority has framed Rules, Regulations and Guidelines to ensure proper care and empathetic treatment of animals in the zoos. In addition, the Central Zoo Authority also undertakes periodic evaluation of zoos, as per the provisions under ‘Recognition of Zoo Rules, 2009’ to ensure the maintenance of standards towards better upkeep and healthcare of animals. *** Annexure-1 ANNEXURE REFERRED TO IN REPLY TO PART (a) OF THE LOK SABHA UNSTARRED QUESTION NO. 2726 REGARDING “ANIMALS IN ZOO” RAISED BY SHRIMATI PRATIMA MONDAL DUE FOR REPLY ON 06.03.2020 *** List of Recognized Zoos in India Sl.No. Zoo Name State Andaman & Nicobar Biological Park, Chidiyatapu 1 Islands 2 Deer Park, Chittoor Andhra Pradesh 3 Deer Park, Kandaleru Andhra Pradesh 4 Indira Gandhi Zoological Park -

Static GK Capsule: 2021
Static GK Capsule: 2021 CONTENTS List of National Parks in India ................................................................................................................................................ 5 List of dams in India ............................................................................................................................................................. 13 List International Airports in India ......................................................................................................................................... 8 Major Ports with key Facts: ................................................................................................................................................... 9 SOME INTERESTING FACTS: .............................................................................................................................................. 10 List of Waterfalls in India ..................................................................................................................................................... 17 List of Waterfalls in World With Country & Area ................................................................................................................ 10 Important Power Plants in India .......................................................................................................................................... 12 List of Thermal Power Plants/Stations in India ..................................................................................................................