Delayed Trait Development and the Convergent Evolution of Shell Kinesis in Turtles Gerardo A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Endoskeletal Origin of the Turtle Carapace
ARTICLE Received 7 Dec 2012 | Accepted 3 Jun 2013 | Published 9 Jul 2013 DOI: 10.1038/ncomms3107 OPEN The endoskeletal origin of the turtle carapace Tatsuya Hirasawa1, Hiroshi Nagashima2 & Shigeru Kuratani1 The turtle body plan, with its solid shell, deviates radically from those of other tetrapods. The dorsal part of the turtle shell, or the carapace, consists mainly of costal and neural bony plates, which are continuous with the underlying thoracic ribs and vertebrae, respectively. Because of their superficial position, the evolutionary origins of these costo-neural elements have long remained elusive. Here we show, through comparative morphological and embryological analyses, that the major part of the carapace is derived purely from endos- keletal ribs. We examine turtle embryos and find that the costal and neural plates develop not within the dermis, but within deeper connective tissue where the rib and intercostal muscle anlagen develop. We also examine the fossils of an outgroup of turtles to confirm that the structure equivalent to the turtle carapace developed independently of the true osteoderm. Our results highlight the hitherto unravelled evolutionary course of the turtle shell. 1 Laboratory for Evolutionary Morphology, RIKEN Center for Developmental Biology, Kobe 650-0047, Japan. 2 Division of Gross Anatomy and Morphogenesis, Department of Regenerative and Transplant Medicine, Niigata University, Niigata 951-8510, Japan. Correspondence and requests for materials should be addressed to T.H. (email: [email protected]). NATURE COMMUNICATIONS | 4:2107 | DOI: 10.1038/ncomms3107 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. All rights reserved. ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms3107 wo types of skeletal systems are recognized in vertebrates, exoskeletal components into the costal and neural plates (Fig. -
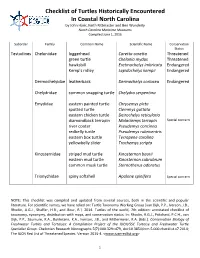
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

A Case of Inversion and Duplication Involving Constitutive Heterochromatin
Genetics and Molecular Biology, 36, 3, 353-356 (2013) Copyright © 2013, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Short Communication Cytogenetic comparison of Podocnemis expansa and Podocnemis unifilis: A case of inversion and duplication involving constitutive heterochromatin Ricardo José Gunski1, Isabel Souza Cunha2, Tiago Marafiga Degrandi1, Mario Ledesma3 and Analía Del Valle Garnero1 1Universidade Federal do Pampa, Campus São Gabriel, São Gabriel, RS, Brazil. 2Secretaria Municipal de Meio Ambiente e Turismo, São Desidério, BA, Brazil. 3Parque Ecológico “El Puma”, Candelaria, Argentina. Abstract Podocnemis expansa and P. unifilis present 2n = 28 chromosomes, a diploid number similar to those observed in other species of the genus. The aim of this study was to characterize these two species using conventional staining and differential CBG-, GTG and Ag-NOR banding. We analyzed specimens of P. expansa and P. unifilis from the state of Tocantins (Brazil), in which we found a 2n = 28 and karyotypes differing in the morphology of the 13th pair, which was submetacentric in P. expansa and telocentric in P. unifilis. The CBG-banding patterns revealed a heterochromatic block in the short arm of pair 13 of P. expansa and an interstitial one in pair 13 of P. unifilis, suggest- ing a pericentric inversion. Pair 14 of P. unifilis showed an insterstitial band in the long arm that was absent in P. expansa, suggesting a duplication in this region. Ag-NORs were observed in the first chromosome pair of both spe- cies and was associated to a secondary constriction and heterochromatic blocks. Keywords: chromosome banding, Ag-NORs, karyotype, chelonids. -

Basal Turtles Shell Bone Histology Indicates Terrestrial Palaeoecology Of
Downloaded from rspb.royalsocietypublishing.org on May 22, 2012 Shell bone histology indicates terrestrial palaeoecology of basal turtles Torsten M Scheyer and P.Martin Sander Proc. R. Soc. B 2007 274, 1885-1893 doi: 10.1098/rspb.2007.0499 Supplementary data "Data Supplement" http://rspb.royalsocietypublishing.org/content/suppl/2009/03/13/274.1620.1885.DC1.ht ml References This article cites 26 articles, 5 of which can be accessed free http://rspb.royalsocietypublishing.org/content/274/1620/1885.full.html#ref-list-1 Article cited in: http://rspb.royalsocietypublishing.org/content/274/1620/1885.full.html#related-urls Receive free email alerts when new articles cite this article - sign up in the box at the top Email alerting service right-hand corner of the article or click here To subscribe to Proc. R. Soc. B go to: http://rspb.royalsocietypublishing.org/subscriptions Downloaded from rspb.royalsocietypublishing.org on May 22, 2012 Proc. R. Soc. B (2007) 274, 1885–1893 doi:10.1098/rspb.2007.0499 Published online 22 May 2007 Shell bone histology indicates terrestrial palaeoecology of basal turtles Torsten M. Scheyer*,† and P. Martin Sander Institute of Palaeontology, University of Bonn, Nussallee 8, 53115 Bonn, Germany The palaeoecology of basal turtles from the Late Triassic was classically viewed as being semi-aquatic, similar to the lifestyle of modern snapping turtles. Lately, this view was questioned based on limb bone proportions, and a terrestrial palaeoecology was suggested for the turtle stem. Here, we present independent shell bone microstructural evidence for a terrestrial habitat of the oldest and basal most well- known turtles, i.e. -

New Records of Mesoclemmys Raniceps (Testudines, Chelidae) for the States of Amazonas, Pará and Rondônia, North Brazil, Including the Tocantins Basin
Herpetology Notes, volume 12: 283-289 (2019) (published online on 18 February 2019) New records of Mesoclemmys raniceps (Testudines, Chelidae) for the states of Amazonas, Pará and Rondônia, North Brazil, including the Tocantins basin Elizângela Silva Brito1,*, Rafael Martins Valadão2, Fábio Andrew G. Cunha3, Cristiane Gomes de Araújo4, Patrik F. Viana5, and Izaias Fernandes Médice6 Of the 58 species of living Chelidae (Rhodin et al., Among the rare species of the genus, Mesoclemmys 2017), 20 are known from Brazil (Costa and Bérnils, raniceps (Gray, 1856), a medium-sized freshwater turtle 2018). Of these, nine occur in the Amazon basin, (approximately 330 mm carapace length - CL; Rueda- including species of the genera Chelus, Mesoclemmys, Almonacid et al., 2007), inhabits streams and flooded Platemys, Phrynops and Rhinemys (Ferrara et al., 2017). forest, but can also be found in rivers, shallow lakes and The genus Mesoclemmys is the most diverse in Brazil, temporary pools in the forest (Vogt, 2008; Ferrara et and five of the eight species of Mesoclemmys in Brazil al., 2017). Mesoclemmys raniceps is relatively easy to occur within the Amazon basin (Souza, 2005; Ferrara identify, especially as an adult. Specimens of this species et al., 2017). Species of genus Mesoclemmys are rare have a large broad head, which is approximately one and inconspicuous when compared to other freshwater quarter of the length of the CL (head width between 23- turtles, and live in hard-to-reach places, to extent that 27%). The head is dark, but may show depigmentation in populations are rarely studied. This genus represents adults, resulting in a lighter color, generally in patches, the least studied among Amazonian turtles (Vogt, 2008; as shown in Figure 2 (af). -

Turtles, All Marine Turtles, Have Been Documented Within the State’S Borders
Turtle Only four species of turtles, all marine turtles, have been documented within the state’s borders. Terrestrial and freshwater aquatic species of turtles do not occur in Alaska. Marine turtles are occasional visitors to Alaska’s Gulf Coast waters and are considered a natural part of the state’s marine ecosystem. Between 1960 and 2007 there were 19 reports of leatherback sea turtles (Dermochelys coriacea), the world’s largest turtle. There have been 15 reports of Green sea turtles (Chelonia mydas). The other two are extremely rare, there have been three reports of Olive ridley sea turtles (Lepidochelys olivacea) and two reports of loggerhead sea turtles (Caretta caretta). Currently, all four species are listed as threatened or endangered under the U.S. Endangered Species Act. Prior to 1993, Alaska marine turtle sightings were mostly of live leatherback sea turtles; since then most observations have been of green sea turtle carcasses. At present, it is not possible to determine if this change is related to changes in oceanographic conditions, perhaps as the result of global warming, or to changes in the overall population size and distribution of these species. General description: Marine turtles are large, tropical/subtropical, thoroughly aquatic reptiles whose forelimbs or flippers are specially modified for swimming and are considerably larger than their hind limbs. Movements on land are awkward. Except for occasional basking by both sexes and egg-laying by females, turtles rarely come ashore. Turtles are among the longest-lived vertebrates. Although their age is often exaggerated, they probably live 50 to 100 years. Of the five recognized species of marine turtles, four (including the green sea turtle) belong to the family Cheloniidae. -

Harvested Testudines
Testudines HARVESTED – Is the species collected by humans? Species Common Name Harvested Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle YF Economically Chelonia mydas is the most important reptile in the world. Its flesh and its eggs serve as an important source of protein in many third-world nations where protein is scarce (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill YF hawksbill flesh and eggs are eaten in many parts of its range (Ernst et al. 1994); YC throughout its range it is hunted for the plates of its shells (Ernst et al. 1994) Lepidochelys kempii Kemp’s ridley or Atlantic ridley YF egg poaching caused the initial decline [in population size] (Delikat 1981; Hall et al. 1983; Lutz and Lutcavage 1989; Ross et al. 1989; Thompson 1989; National Research Council 1990; Caillouet et al. 1991) Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback YF gathering the eggs for both commerce and personal consumption (Ernst et al. 1994) Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle YF the flesh of the snapping turtle is tasty and eaten throughout the range…fresh eggs are edible if fried (Ernst et al. 1994) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk Chrysemys p. marginata midland painted turtle Unk Clemmys guttata spotted turtle YP (Lovich and Jaworski 1988; Lovich 1989; J. Harding, pers. comm.) Clemmys insculpta wood turtle YP (Ernst et al. 1994) Clemmys muhlenbergii bog turtle YP (D.E. Collins 1990); YF eaten by people (Ernst et al. 1994) Deirochelys r. -

Register 2 0
© T E R R A R I S T I K - F A C H M A G A Z I N R E G I S T E R 2 0 1 0 1 REPTILIA-Register 2010 Terrarienpraxis 85, Oktober/November, 15(5): 38–45. BIRTEL, Andreas (2010): Ein Gewächshaus für Grüne Leguane und Pan- GEHRING, Philip-Sebastian, Maciej PABIJAN, Fanomezana M. RATSOAVI- therchamäleons. – Nr. 84, August/September 2010, 15(4): 44–45. NA, Jörn KÖHLER, Konrad MEBERT & Frank GLAW (2010): Calum- BRAUN, Sandra (2010): Bau eines naturnahen Schauterrariums für ein ma tarzan. Eine neue Chamäleonart aus Madaskar braucht dringend Jemenchamäleon. – Nr. 84, August/September 2010, 15(4): 40–43. Hilfe! – Nr. 86, Dezember 2010/Januar 2011, 15(6): 60–64. FRÖMBERG, Carsten (2010): Gestaltung von Verstecken unter Nutzung GUTSCHE, Alexander (2010): Mehrere Amphibien- und Reptilienarten von Latex-Bindemittel. – Nr. 84, August/September 2010, 15(4): neu in das Washingtoner Artenschutzabkommen (CITES) aufge- 36–38. nommen. – Nr. 83, Juni/Juli 2010, 15(3): 3–8. LONGHITANO, Filip (2010): Vorteile der Rackhaltung. – Nr. 84, August/ JACHAN, Georg (2010): Pfl ege und Vermehrung der Usambara-Busch- September 2010, 15(4): 34–35. viper Atheris ceratophora. – Nr. 83, Juni/Juli 2010, 15(3): 58–68. SCHLÜTER, Uwe (2010): Ernährung nord- und westafrikanischer Wara- KOCH, André (2010): Bestialische Behandlung indonesischer Großrep- ne in der Natur und bei Terrarienhaltung. – Nr. 86, Dezember 2010/ tilien für westliche Luxusprodukte. – Nr. 86, Dezember 2010/Januar Januar 2011, 15(6): 36–45. 2011, 15(6): 3–6. SCHMIDT, Dieter (2010): Naturterrarium oder Heimtierkäfi g? – Nr. 84, KOCH, Claudia (2010): Geheimnisvolles Peru. -

Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships
438 Article 438 by Saswati Bandyopadhyay1* and Sanghamitra Ray2 Gondwana Vertebrate Faunas of India: Their Diversity and Intercontinental Relationships 1Geological Studies Unit, Indian Statistical Institute, 203 B. T. Road, Kolkata 700108, India; email: [email protected] 2Department of Geology and Geophysics, Indian Institute of Technology, Kharagpur 721302, India; email: [email protected] *Corresponding author (Received : 23/12/2018; Revised accepted : 11/09/2019) https://doi.org/10.18814/epiiugs/2020/020028 The twelve Gondwanan stratigraphic horizons of many extant lineages, producing highly diverse terrestrial vertebrates India have yielded varied vertebrate fossils. The oldest in the vacant niches created throughout the world due to the end- Permian extinction event. Diapsids diversified rapidly by the Middle fossil record is the Endothiodon-dominated multitaxic Triassic in to many communities of continental tetrapods, whereas Kundaram fauna, which correlates the Kundaram the non-mammalian synapsids became a minor components for the Formation with several other coeval Late Permian remainder of the Mesozoic Era. The Gondwana basins of peninsular horizons of South Africa, Zambia, Tanzania, India (Fig. 1A) aptly exemplify the diverse vertebrate faunas found Mozambique, Malawi, Madagascar and Brazil. The from the Late Palaeozoic and Mesozoic. During the last few decades much emphasis was given on explorations and excavations of Permian-Triassic transition in India is marked by vertebrate fossils in these basins which have yielded many new fossil distinct taxonomic shift and faunal characteristics and vertebrates, significant both in numbers and diversity of genera, and represented by small-sized holdover fauna of the providing information on their taphonomy, taxonomy, phylogeny, Early Triassic Panchet and Kamthi fauna. -

Reproductive Physiology of Nesting Leatherback Turtles (Dermochelys Coriacea) at Las Baulas National Park, Costa Rica
Chelolliall COllserwuioll (lnd Biology, 1996,2(2):230- 236 © 1996 by Chelonian Research Foundation Reproductive Physiology of Nesting Leatherback Turtles (Dermochelys coriacea) at Las Baulas National Park, Costa Rica i 2 3 4 DAVID C. ROSTAL , FRANK V. PALADIN0 , RHONDA M. PATTERSON , AND JAMES R. SPOTILA I Department of Biology, Georgia Southern Uni versity, Statesboro, Georgia 30460 USA [Fax: 912-681-0845; E-mail: [email protected]}; 2Department of Biology, Indiana-Purdue University, Fort Wayne, Indiana 46805 USA ; 3Department of Biology, Texas A&M University, College Station, Texas 77843 USA ; 4Department o{Bioscience and Biotechnology, Drexel University, Philadelphia, Pennsylvania 19104 USA ABSTRACT. - The reproductive physiology of nesting leatherback turtles (Dermochelys coriacea) was studied at Playa Grande, Costa Rica, from 1992 to 1994 during November, December, and January. Ultrasonography indicated that 82 % of nesting females had mature preovulatory ovaries in November, 40 % in December, and 23 % in January. Mean follicular diameter was 3.33 ±0.02 cm and did not vary significantly through the nesting season. Plasma testosterone and estradiol levels measured by radioimmunoassay correlated strongly with reproductive condition. No correlation, however, was observed between reproductive condition and plasma progesterone levels. Testoster one declined from 2245 ± 280 pglml at the beginning of the nesting cycle to 318 ± 89 pglml at the end of the nesting cycle. Estradiol declined in a similar manner. Plasma calcium levels were constant throughout the nesting cycle. Vitellogenesis appeared complete prior to the arrival of the female at the nesting beach. Dermochelys coriacea is a seasonal nester displaying unique variations (e.g., 9 to 10 day internesting interval, yolkless eggs) on the basic chelonian pattern. -

Ecol 483/583 – Herpetology Lab 11: Reptile Diversity 3: Testudines and Crocodylia Spring 2010
Ecol 483/583 – Herpetology Lab 11: Reptile Diversity 3: Testudines and Crocodylia Spring 2010 P.J. Bergmann & S. Foldi Lab objectives The objectives of today’s lab are to: 1. Familiarize yourselves with extant diversity of the Testudines and Crocodylia. 2. Learn to identify species of Testundines that live in Arizona. Today's lab is the final lab on "reptile" diversity, and will introduce you to the Testudines, or turtles and tortoises, Crocodylia. Although there are no crocodylians in Arizona and the Testudine diversity is lower than that of the lizards or snakes, there is still a fair amount of material to learn, so use your time wisely. Tips for learning the material At this point in the course, your skills for learning herp diversity should be well honed, so continue with the strategies you have already learned during the semester. Learn how to differentiate the three major clades of Crocodylians, and the more numerous Testudine clades. There is another keying exercise in this lab, focusing on the Testudines. 1 Ecol 483/583 – Lab 11: Testudines & Crocs 2010 Exercise 1: Testudines (Modified from Bonine & Foldi 2008; Bonine, Dee & Hall 2006; Edwards 2002; Prival 2000) General information Turtles are probably the most instantly recognizable groups of all reptiles because of their shell and the ability to withdraw their heads and limbs into this protective structure. Turtles are a monophyletic group comprising the order Testudines also called Chelonia . Testudines is the term used to denote all members of the order (extant and extinct) whereas Chelonia is often used to denote extant turtles. -

Evolutionary Origin of the Turtle Shell
Current Biology 23, 1113–1119, June 17, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.05.003 Report Evolutionary Origin of the Turtle Shell Tyler R. Lyson,1,2,3,* Gabe S. Bever,4,5 Torsten M. Scheyer,6 debated throughout the 20th and early 21st centuries (see Allison Y. Hsiang,1 and Jacques A. Gauthier1,3 [16]) with support falling largely along disciplinary lines [3–9, 1Department of Geology and Geophysics, Yale University, 17–25]. Paleontological explanations relied heavily on the 210 Whitney Avenue, New Haven, CT 06511, USA composite model [19–25], but their efficacy was hampered 2Department of Vertebrate Zoology, National Museum of by the large morphological gap separating the earliest, fully Natural History, Smithsonian Institution, Washington, shelled turtles (e.g., Proganochelys quenstedti [26]) from all DC 20560, USA other known groups. In contrast, developmental biologists 3Division of Vertebrate Paleontology, Yale Peabody Museum promoted the de novo model and viewed the lack of clear tran- of Natural History, New Haven, CT 06511, USA sitional fossils as support for a rapid evolution of the shell, 4New York Institute of Technology, College of Osteopathic perhaps coincident with the appearance of a bone morphoge- Medicine, Old Westbury, NY 11568, USA netic protein (BMP) developmental pathway critical to shell 5Division of Paleontology, American Museum of Natural construction in modern turtles [3–9, 27]. The lack of osteo- History, New York, NY 10024, USA derms in the recently discovered stem turtle Odontochelys 6Pala¨ ontologisches Institut und Museum, Universita¨ tZu¨ rich, semitestacea [2] strongly supports the de novo model of shell Karl-Schmid-Strasse 4, 8006 Zu¨rich, Switzerland origination and liberates the paleontological search for the even deeper history of the turtle stem from its previously self-imposed constraint of osteoderm-bearing forms.