Ecological Profile of Sharavathi River Basin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Situation Report Nature of Hazard: Floods Current Situation
India SITUATION REPORT NATURE OF HAZARD: FLOODS In Maharashtra Bhandara and Gondia were badly affected but situation has improved there. Andhra Pradesh situation is getting better in Khamam, East and West Godavary districts. Road connectivity getting restored and Communication is improving. People from the camps have started returning back. Flood Situation is under control as the Rivers in Andhra Pradesh are flowing at Low Flood Levels. In Surat situation is getting much better as Tapi at Ukai dam is flowing with falling trend In Maharashtra River Godavari is flowing below the danger level. In Maharashtra Konkan and Vidharbha regions have received heavy rainfall. Rainfall in Koyna is recorded at 24.9mm and Mahableshwar 18mm in Santa Cruz in Mumbai it is 11mm. The areas which received heavy rainfall in last 24 hours in Gujarat are Bhiloda, Himatnagar and Vadali in Sabarkantha district, Vav and Kankrej in Banskantha district and Visnagar in Mehsana. IMD Forecast; Yesterday’s (Aug16) depression over Orissa moved northwestwards and lay centred at 0830 hours IST of today, the 17th August, 2006 near Lat. 22.00 N and Long. 83.50 E, about 100 kms east of Champa. The system is likely to move in a northwesterly direction and weaken gradually. Under its influence, widespread rainfall with heavy to very heavy falls at few places are likely over Jharkhand and Chhattisgarh during next 24 hours. Widespread rainfall with heavy to very heavy falls at one or two places are also likely over Orissa, Vidarbha and east Madhya Pradesh during the same period -

Career Profile Of
Career Profile of Er. E. Sreedharan Chairman & Managing Director, Kokan Railway Corporation Ltd Recipient of S.B. Joshi Memorial Award for Bridge & Structural Engineering for the year 1995, cited by Alumni Association of College of Engineering, Pune Date of Birth: • 12th June, 1932 Educational Qualification and Training: • BE (Civil), Govt. College of Engg, Kakinada, Kerala in April 1953 Professional Experience and Achievements: • Held a number of positions as Assistant Engineer, Executive Engineer, Divisional Engineer and Deputy Chief Engineer on the Southern and South Eastern Railways. • In-charge of new line constructions such as Quilon-Ernakulam metre gauge line, Mangalore-Hassan railway line, a number of doubling projects, bridge and tunnel projects and also maintenance of permanent ways in Palghat, Hubli and Vijaywada Divisions. • Restored the Pamban Railway Bridge in 46 days, 125 spans of which were washed away in a tidal wave in December 1963. • As Dy. Chief Engineer, in-charge of investigation, planning and design of the first ever Metro in the country, viz. at Calcutta from 1970 to 1975. • Worked as Divisional Supdt., Mysore Division, Southern Railway and as Additional Chief Engineer (Track), Southern Railway from 1976 to 1979. • As Chief Engineer (Construction), Eastern Railway in March 1979, in- charge of all the major Railway Construction Projects on that Railway. • Worked as Chief Engineer (Construction), Southern Railway, in-charge of all maojot projects on that Railway from 1981 to 1985. • In February1986, as Chief Administrative Office (Construction), Central Railway, in charge of all the major construction activities and Metropolitan Transport Project on that Railway. • In June 1980, in-charge of organizing the preliminary works for the prestigious Konkan Railway and subsequently, as Chairman and 1 Managing Director of the Konkan Railway Corporation Ltd in October 1990. -

Hampi, Badami & Around
SCRIPT YOUR ADVENTURE in KARNATAKA WILDLIFE • WATERSPORTS • TREKS • ACTIVITIES This guide is researched and written by Supriya Sehgal 2 PLAN YOUR TRIP CONTENTS 3 Contents PLAN YOUR TRIP .................................................................. 4 Adventures in Karnataka ...........................................................6 Need to Know ........................................................................... 10 10 Top Experiences ...................................................................14 7 Days of Action .......................................................................20 BEST TRIPS ......................................................................... 22 Bengaluru, Ramanagara & Nandi Hills ...................................24 Detour: Bheemeshwari & Galibore Nature Camps ...............44 Chikkamagaluru .......................................................................46 Detour: River Tern Lodge .........................................................53 Kodagu (Coorg) .......................................................................54 Hampi, Badami & Around........................................................68 Coastal Karnataka .................................................................. 78 Detour: Agumbe .......................................................................86 Dandeli & Jog Falls ...................................................................90 Detour: Castle Rock .................................................................94 Bandipur & Nagarhole ...........................................................100 -

Shankar Ias Academy Test 18 - Geography - Full Test - Answer Key
SHANKAR IAS ACADEMY TEST 18 - GEOGRAPHY - FULL TEST - ANSWER KEY 1. Ans (a) Explanation: Soil found in Tropical deciduous forest rich in nutrients. 2. Ans (b) Explanation: Sea breeze is caused due to the heating of land and it occurs in the day time 3. Ans (c) Explanation: • Days are hot, and during the hot season, noon temperatures of over 100°F. are quite frequent. When night falls the clear sky which promotes intense heating during the day also causes rapid radiation in the night. Temperatures drop to well below 50°F. and night frosts are not uncommon at this time of the year. This extreme diurnal range of temperature is another characteristic feature of the Sudan type of climate. • The savanna, particularly in Africa, is the home of wild animals. It is known as the ‘big game country. • The leaf and grass-eating animals include the zebra, antelope, giraffe, deer, gazelle, elephant and okapi. • Many are well camouflaged species and their presence amongst the tall greenish-brown grass cannot be easily detected. The giraffe with such a long neck can locate its enemies a great distance away, while the elephant is so huge and strong that few animals will venture to come near it. It is well equipped will tusks and trunk for defence. • The carnivorous animals like the lion, tiger, leopard, hyaena, panther, jaguar, jackal, lynx and puma have powerful jaws and teeth for attacking other animals. 4. Ans (b) Explanation: Rivers of Tamilnadu • The Thamirabarani River (Porunai) is a perennial river that originates from the famous Agastyarkoodam peak of Pothigai hills of the Western Ghats, above Papanasam in the Ambasamudram taluk. -

RTM-February -2020 Magazine
INSIGHTSIAS IA SIMPLIFYING IAS EXAM PREPARATION RTM COMPILATIONS PRELIMS 2020 FEBRUARY 2020 www.insightsactivelearn.com | www.insightsonindia.com Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 2 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Telegram: https://t.me/insightsIAStips 3 Youtube: https://www.youtube.com/channel/UCpoccbCX9GEIwaiIe4HLjwA Revision Through MCQs (RTM) Compilation (February 2020) Table of Contents RTM- REVISION THROUGH MCQS – 1st Feb-2020 ............................................................... 5 RTM- REVISION THROUGH MCQS – 3st Feb-2020 ............................................................. 10 RTM- REVISION THROUGH MCQS – 5th Feb-2020 ............................................................. 16 RTM- REVISION THROUGH MCQS – 6th Feb-2020 ............................................................. 22 RTM- REVISION THROUGH MCQS – 7th Feb-2020 ............................................................. 28 RTM- REVISION THROUGH MCQS – 8th Feb-2020 ............................................................. 34 RTM- REVISION THROUGH MCQS – 10th Feb-2020 ........................................................... 40 RTM- REVISION THROUGH MCQS – 11th Feb-2020 ........................................................... 45 RTM- REVISION THROUGH MCQS – 12th Feb-2020 ........................................................... 52 RTM- REVISION THROUGH MCQS – 13th Feb-2020 .......................................................... -
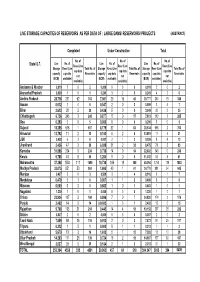
Live Storage Capacities of Reservoirs As Per Data of : Large Dams/ Reservoirs/ Projects (Abstract)
LIVE STORAGE CAPACITIES OF RESERVOIRS AS PER DATA OF : LARGE DAMS/ RESERVOIRS/ PROJECTS (ABSTRACT) Completed Under Construction Total No. of No. of No. of Live No. of Live No. of Live No. of State/ U.T. Resv (Live Resv (Live Resv (Live Storage Resv (Live Total No. of Storage Resv (Live Total No. of Storage Resv (Live Total No. of cap data cap data cap data capacity cap data Reservoirs capacity cap data Reservoirs capacity cap data Reservoirs not not not (BCM) available) (BCM) available) (BCM) available) available) available) available) Andaman & Nicobar 0.019 20 2 0.000 00 0 0.019 20 2 Arunachal Pradesh 0.000 10 1 0.241 32 5 0.241 42 6 Andhra Pradesh 28.716 251 62 313 7.061 29 16 45 35.777 280 78 358 Assam 0.012 14 5 0.547 20 2 0.559 34 7 Bihar 2.613 28 2 30 0.436 50 5 3.049 33 2 35 Chhattisgarh 6.736 245 3 248 0.877 17 0 17 7.613 262 3 265 Goa 0.290 50 5 0.000 00 0 0.290 50 5 Gujarat 18.355 616 1 617 8.179 82 1 83 26.534 698 2 700 Himachal 13.792 11 2 13 0.100 62 8 13.891 17 4 21 J&K 0.028 63 9 0.001 21 3 0.029 84 12 Jharkhand 2.436 47 3 50 6.039 31 2 33 8.475 78 5 83 Karnatka 31.896 234 0 234 0.736 14 0 14 32.632 248 0 248 Kerala 9.768 48 8 56 1.264 50 5 11.032 53 8 61 Maharashtra 37.358 1584 111 1695 10.736 169 19 188 48.094 1753 130 1883 Madhya Pradesh 33.075 851 53 904 1.695 40 1 41 34.770 891 54 945 Manipur 0.407 30 3 8.509 31 4 8.916 61 7 Meghalaya 0.479 51 6 0.007 11 2 0.486 62 8 Mizoram 0.000 00 0 0.663 10 1 0.663 10 1 Nagaland 1.220 10 1 0.000 00 0 1.220 10 1 Orissa 23.934 167 2 169 0.896 70 7 24.830 174 2 176 Punjab 2.402 14 -

LIST of INDIAN CITIES on RIVERS (India)
List of important cities on river (India) The following is a list of the cities in India through which major rivers flow. S.No. City River State 1 Gangakhed Godavari Maharashtra 2 Agra Yamuna Uttar Pradesh 3 Ahmedabad Sabarmati Gujarat 4 At the confluence of Ganga, Yamuna and Allahabad Uttar Pradesh Saraswati 5 Ayodhya Sarayu Uttar Pradesh 6 Badrinath Alaknanda Uttarakhand 7 Banki Mahanadi Odisha 8 Cuttack Mahanadi Odisha 9 Baranagar Ganges West Bengal 10 Brahmapur Rushikulya Odisha 11 Chhatrapur Rushikulya Odisha 12 Bhagalpur Ganges Bihar 13 Kolkata Hooghly West Bengal 14 Cuttack Mahanadi Odisha 15 New Delhi Yamuna Delhi 16 Dibrugarh Brahmaputra Assam 17 Deesa Banas Gujarat 18 Ferozpur Sutlej Punjab 19 Guwahati Brahmaputra Assam 20 Haridwar Ganges Uttarakhand 21 Hyderabad Musi Telangana 22 Jabalpur Narmada Madhya Pradesh 23 Kanpur Ganges Uttar Pradesh 24 Kota Chambal Rajasthan 25 Jammu Tawi Jammu & Kashmir 26 Jaunpur Gomti Uttar Pradesh 27 Patna Ganges Bihar 28 Rajahmundry Godavari Andhra Pradesh 29 Srinagar Jhelum Jammu & Kashmir 30 Surat Tapi Gujarat 31 Varanasi Ganges Uttar Pradesh 32 Vijayawada Krishna Andhra Pradesh 33 Vadodara Vishwamitri Gujarat 1 Source – Wikipedia S.No. City River State 34 Mathura Yamuna Uttar Pradesh 35 Modasa Mazum Gujarat 36 Mirzapur Ganga Uttar Pradesh 37 Morbi Machchu Gujarat 38 Auraiya Yamuna Uttar Pradesh 39 Etawah Yamuna Uttar Pradesh 40 Bangalore Vrishabhavathi Karnataka 41 Farrukhabad Ganges Uttar Pradesh 42 Rangpo Teesta Sikkim 43 Rajkot Aji Gujarat 44 Gaya Falgu (Neeranjana) Bihar 45 Fatehgarh Ganges -

B. Pre-Feasibility Report (Extracts of DPR) 1
Water Re-circulation and Environmental Sustainability Pre-feasibility Report Project for Jog Falls, Shivamogga District Karnataka B. Pre-feasibility report (extracts of DPR) 1. Executive Summary x The proposed project is a water recirculation project by pumping 400 cusecs of water in the summer months (8 months, November - June) from the storage pond near MGHE to the weir proposed to be constructed in the upstream of Sita Katte bridge and use the reversible flow in the same pipeline for generating power for 4 months (July-October). x The water from the weir will be released to the falls in the summer months (for about 8 months from November to June). This pumped water, drops over the falls for a maximum of about 8 hours each day for 8 months. The discharged water at the upstream will have a natural distribution through cascades before the drop in the falls. x In rainy season (June to October), the same pipeline would be used to generate power. The pump house/power house will be located in the area opposite side of Mahatma Gandhi Hydro Electric (MGHE) power house adjacent to Ambuthirtha reservoir/MGHE powerhouse tail water pond. The reservoir has sufficient storage capacity. x The water is pumped directly from the downstream storage pond through two pipes of 1600 mm diameter, which passes through a tunnel near Bombay guest house. The total distance to be covered from the pump house to the foot of the anicut near the Sita Katte bridge shall be approximately 3210 m. x The power house with two generators will be with an installed capacity for generation of power of 16.61 MW each in monsoon months (totalling to 47.84 million units) and the power consumed in non-monsoon months (pump mode) would be 24.74 MW each amounting to 47.80 Million units. -

Assessment of Water Quality Changes in Krishna River of Andhrap Radesh Through Geoinformatics
International Journal of Recent Technology and Engineering (IJRTE) ISSN: 2277-3878, Volume-7, Issue-6C2, April 2019 Assessment of Water Quality Changes in Krishna River of Andhrap radesh Through Geoinformatics Lakshman Kumar.C.H, D. Satish Chandra, S.S.Asadi Abstract--- Pancha Boothas are Life and Death for the are permissible in river water but exceed their level its Environment. In that any one is Disrupted that can be Escort to causes several diseases for users and Toxic elements, excess the danger of environment. Water is the one of the Pancha nutrients create vadose zones in river courses [5]. Most of Boothas. Quality of the water is very crucial in the present and the assured irrigation in India is surface water of rivers. It is future users. Natural issues and manmade activities are depending on the water quality. The ratio of transportation of essential to monitor and assess the water quality in the fresh water in liquid form to covert useless form is 70%. The Krishna river course. ratio of sedimentation is also one of the parameter of the water quality, if changes are happen in sedimentation the quality of the Notations: water also changes. The causes of water pollution source are GDSQ: Gauge Discharge Sediment and Water Quality many, of which sewage discharge, industrial effluents, agricultural effluents and several man made activities are play a GDQ : Gauge Discharge Water Quality key role on water quality. The total percentage of water in the pH : Potential of Hydrogen world is 97% in Oceans and reaming 3% of water in form of EC : Electric Conductivity glaciers, in which the consumption of water quantity is in form of CO3 : Carbonate surface and subsurface water bodies. -

History and Culture of Karnataka (From Early Times to 1336)
History and Culture of Karnataka (From Early Times to 1336) Programme ಕಾರ್ಯ响ರ ಮ BA Subject 풿ಷರ್ History and Archaeology Semester �ಕ್ಷ貾ವ鲿 V University 풿ಶ್ವ 풿ದ್ಯಾ ಲರ್ Karnatak University, Dharwad Session ಅವ鲿 7 Title : Geographical Features of Karnataka Sub Title: Introduction, Classification- Importance of Geographical features Learning Objectives To enable the students to understand the Geographical features of Karnataka Session Out Comes Students will be able to express their view on Geographical features of Karnataka Introduction • Karnataka State is situated in between 11.30 to 18.48 Northern latitude and 74.12 to 78.50 East longitude, • Karnataka is surrounded by Maharashtra in North, Goa in Northwest, Tamilnadu & Keral in South, Andhara Pradesh & Telengana in East. • Karnataka is 2000 feet above sea level. • Present Karnataka is divided in to 30 Districts 230 Talukas 29733 Villages. Introduction……. • The length of the state is 770 km and breadth is 400 km • Total extent of the State is 1,92,204 sq. km • Krishna, Bhima, Tungabhadra, Malaprabha, Ghatprabha, Kali, Sharavati, Varadha, Kaveri, Netravati, Arkavati, Aghanashini etc. are the important rivers in the State. • The region where two rivers joins is called as Doab. Shorapur Doab in Yadgiri district where river Bhima joins the Krishna. Raichur Doab where river Tungabhadra joins Krishna, the plateau of Raichur Doab & Tungabhdra referred as Rayalaseema. Introduction……. • Origin of the Name : Karnataka,Karnata, Kannada refers to a region and language. • Kar+nadu= land of black soil. • Temil epic Shilappadhikaram & Tolkappiyam refers as Karunat= High land or Big land • Mahabharat Sabhaparva & Bhishmaparva – Karnataka. • Sudraka-Mrichchakatika & varahamihira’s Brihatsamhita refers- Karnataka. -

Team ( For) Team ( Against) Topic Slot JUDGES Mississipi
Team ( for) Team ( Against) Topic Slot JUDGES Are parents to be held responsible for the actions of their Mississipi - thames Kaveri children? 10:00-10:30 Aparna-Ananya Should MLAs and MPs should have a minimum level of Yamuna - tapi Krishna educational qualification? 17 apil- 10:00-10:30 prashasti-jay sandhiya- Mahanadhi Tigris Is Indian culture decaying? 5:00- 5:30 shailendra Should we make cartoons and TV a part of the educational Koshi Narmada process in elementary school? 10:45-11:15 shrishty-shivam Homework at school: should be banned or it is an essential Rupnarayan Sindhu part of our studies that teaches us to work independently. 11:30-12:00 Aparna-Ananya Jordan Jhelum - Indus Social media has improved human communication and reach. 11:30-12:00 prashasti-jay Patriotism is doing more harm than good when it comes to sandhiya- Danube Betwa International relations. 12:15-12:45 shailendra Government shouldn't have the access to personal information Colorado Brahmaputra of citizens through the linking of Adhaar. 12:15-12:45 shrishty-shivam Alknanda Tista Does 'NOTA' option in elections really make sense? 1:00-1:30 Aparna-Ananya Tests on animals: should animals be used for scientific Godavari Shinano achievements 1:00-1:30 Prashasti-jay sandhiya- Amazon Irtysh Film versions are never as good as the original books. 1:30-2:00 shailendra Sutlej Gandak Zoos should be banned. 1:30-2:00 shrishty-shivam Ganga Umngot Online system of education is a boon than a bane. 2:00-2:30 Aparna-Ananya zambezi- WILD CARD Team Team Winning Slot Jugdes Topics Social media comments should be Mississipi + Thames Kaveri Kaveri (A) 12:00- 12:30 p.m. -

Geographical Features of Karnataka
Class : B.A 5th Semester Subject : History & Archaeology Title of the Paper : History and Culture of Karnataka(From Early Times to 1336) Paper II Optional Session: 7,8 & 9. Topic : Geographical Features of Karnataka. __________________________________________________________________________________ Introduction Karnataka State is situated in between 11.30 to 18.48 Northern latitude and 74.12 to 78.50 East longitude, Karnataka is surrounded by Maharashtra in North, Goa in Northwest, Tamilnadu & Keral in South, Andhara Pradesh & Telengana in East. Karnataka is 2000 feet above sea level. Present Karnataka is divided in to 30 Districts 230 Talukas 29733 Villages. The length of the state is 770 km and breadth is 400 km total extent of the State is 1,92,204 sq. km The main rivers of Karnataka is Krishna, Bhima, Tungabhadra, Malaprabha, Ghatprabha, Kali, Sharavati, Varadha, Kaveri, Netravati, Arkavati, Aghanashini etc. are the important rivers in the State. The region where two rivers joins is called as Doab. Shorapur Doab in Yadgiri district where river Bhima joins the Krishna. Raichur Doab where river Tungabhadra joins Krishna, the plateau of Raichur Doab & Tungabhdra referred as Rayalaseema. Geographical Classification of Karnataka 1. Coastal region 2. Sahyadri Mountains /Western Ghats 3. Northern Plain 4. Southern Plain Importance of Geographical Features : Richard Hakluyat, pointed out that “The Geography & Chronology are the Sun & Moon, the right and left eye of History”. Human history in a region is shaped by the physical features. The growth of civilization is depend upon the climate, fertility of soil, natural barriers. Geographically Karnataka is one of the oldest part of Deccan plateau. The history and culture of Karnataka has been molded by the Geographical features.