Incubation Rhythms of Ring-Necked Ducks ’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
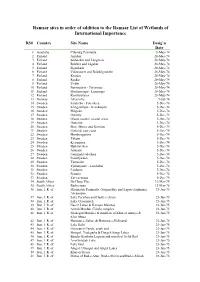
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Delta Marsh Wildlife Management Area
Delta Marsh Wildlife Management Area Land Description Delta Marsh is an extensive freshwater coastal wetland at the southern end of Lake Manitoba. It stretches from Lynchs Point in the west to St. Ambroise in the east. The protected portion of the Delta Marsh Wildlife Management Area encompasses 8,125 hectares. These protected lands are free from logging, mining, hydroelectric, oil and gas development as well as other activities that could significantly and adversely affect habitat. The total size of the wildlife management area is 11,000 hectares. Outstanding Features The Delta Marsh in south central Manitoba is one of the largest and most important marshes in the Canadian prairies, occupying approximately 18,000 hectares at the south end of Lake Manitoba. In 1982, the Delta Marsh was listed as a wetland of international importance under the International Union for the Conservation of Nature (IUCN) Ramsar Convention. It is recognized provincially as a “Manitoba Heritage Marsh” and nationally as an Important Bird Area due to its significance for waterfowl and neotropical migrants. The marsh formed between 2,500 and 4,500 years ago when the ancient Assiniboine River flowed into Lake Manitoba. It is composed of a network of interconnected shallow bays separated from Lake Manitoba by a wooded barrier beach. White-tailed deer are common on the lands bordering the marsh, with gray partridge and sharp-tailed grouse seen year round as well. Common furbearers include coyote, red fox, beaver and muskrat. Western grebes, pelicans and cormorants are found in the marsh. Although traditionally noted for its abundance of waterfowl, such as canvasbacks, lesser scaup and mallards, Delta Marsh is also a critical site for migrating songbirds. -

2020 Canadian NAWMP Report
September 2020 nawmp.wetlandnetwork.ca HabitatMatters 2020 Canadian NAWMP Report “Come Spring – Northern Pintail” from the 2020 Canadian Wildlife Habitat Conservation Stamp series. Artist: DJ Cleland-Hura North American Waterfowl Management Plan —— Plan nord-américain de gestion de la sauvagine —— Plan de Manejo de Aves Acuáticas Norteamérica WE WANT TO HEAR FROM YOU Fill out our survey by December 31, 2020, for a chance at winning a prize. https://www.surveymonkey.com/r/HabitatMatters https://www.surveymonkey.com/r/Habitats_Canadiens TableContents of 1 About the NAWMP 2 National Overview 2 Accomplishments 3 Expenditures and Contributions 4 Celebrating Ecological Restoration Successes 6 Habitat Joint Ventures 7 Pacific Birds Habitat Joint Venture 13 Canadian Intermountain Joint Venture 17 Prairie Habitat Joint Venture 22 Eastern Habitat Joint Venture 28 Species Joint Ventures 29 Black Duck Joint Venture 32 Sea Duck Joint Venture 34 Arctic Goose Joint Venture 36 Partners About the NAWMP Northern Pintails. The North American Waterfowl Management Plan (NAWMP) Ducks Unlimited Canada is an international partnership to restore, conserve and protect waterfowl populations and associated habitats Mexico became a signatory to the NAWMP with its update through management decisions based on strong biological in 1994. As a result, the NAWMP partnership extends across foundations. The ultimate goal is to achieve abundant and North America, working at national and regional levels on a resilient waterfowl populations and sustainable landscapes. variety of waterfowl and habitat management issues. The NAWMP engages the community of users and supporters Since its creation, the NAWMP’s partners have worked to committed to conserving and valuing waterfowl and wetlands. -

B + 1 ,EE;Trr;Ment Environnement Fam*~1Coz’Rj3 --Hiericm Wérhdf Canada Canadian Wildlife Service Canadien Service De La Faune Printed September 1996 Ottawa, Ontario
STRATEGIC OVERVIEW OF THE CANADIAN RAMSAR PROGRAM 7 1 0 B + 1 ,EE;trr;ment Environnement fAm*~1COz’rj3 --hiericm WérhdF Canada Canadian Wildlife Service canadien Service de la faune Printed September 1996 Ottawa, Ontario This document, Strategic Overview of the Canadian Ramsar Program, has been produced as a discussion paper for Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions. The paper provides a general overview of the development, current status and opportunities for the future direction of the Ramsar program in Canada . Comments and suggestions on the content of this paper are welcome at the address below. Copies of this paper are available from: ® Habitat Conservation Division Canadian Wildlife Service Environment Canada Ottawa, Ontario K1 A OH3 it Phone: (819) 953-0485 Fax: (819) 994-4445 Également disponible en français. NpA ENTq4 0~ec 50% recycled paper including 10% poet- wneumer fibre. %ue 0e 50 p" 100 CIO pepier recyclé dwrt 10 p. 100 de fibres post . consOmmetiorn STRATEGIC OVERVIEW OF THE CANADIAN RAMSAR PROGRAM Prepared by: Clayton D.A. Rubec and Manjit Kerr-Upal September 1996 Habitat Conservation Division Canadian Wildlife Service Environment Canada TABLE OF CONTENTS The Ramsar Convention . .1 Ramsar in North America . : . 2 Ramsar in Canada . .2 Canada's Ramsar Database. .. .3 Distribution of Canada's Ramsar Sites . .. ... .. .. .. .. .. .. .. .3 Jurisdictional Distribution . .. .. 4 Ecozonal and Ecoregional Distributicn . .- . .- 4 Wetland Regions Distribution . 7 Wetland Classification Analysis . 8 Selection Criteria . 9 Management of Canadian Ramsar Sites . 9 Responsible Authorities for Ramsar in Canada . 13 Considerations for a National Ramsar Committee for Canada . -

NOMINATION and LISTING of WETLANDS of INTERNATIONAL IMPORTANCE in CANADA Procedures Manual
NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C..D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document. Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions . This report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance . Copies of this Report are available from: Wildlife Conservation Branch Canadian Wildlife Service Environment Canada Ottawa, Ontario _ K1A OH3 NOMINATION AND LISTING OF WETLANDS OF INTERNATIONAL IMPORTANCE IN CANADA Procedures Manual Revised Edition November 1999 Compiled by C .D.A. Rubec Wildlife Conservation Branch Canadian Wildlife Service Environment Canada First Edition February 1994 Revised Edition November 1999 This document Nomination and Listing of Wetlands of International Importance in Canada: Procedures Manual has been produced as one of a series of Canadian Ramsar Network Reports . These documents provide information and general guidance to Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions. This .report is designed to be updated on a periodic basis as new Ramsar site nomination or designation criteria and procedures are adopted by the Contracting Parties to the Convention on Wetlands of International Importance . -

DELTA MARSH, MANITOBA Information Sheet on Ramsar Wetlands
DELTA MARSH, MANITOBA Information Sheet on Ramsar Wetlands Effective Date of Information: The information provided is taken from the List of Canadian Wetlands Designated as of International Importance, May 1982 and updated by the Canadian Wildlife Service in March 1993. Reference: 4CA004 Name and Address of Compiler: Environmental Conservation Branch, Environment Canada, Twin Atria, 2nd Floor, 4999 - 98th Avenue, Edmonton, Alberta, T6B 2X3. Date of Ramsar Designation: 24 May 1982. Geographical Coordinates: 5005'N., 9800'W. General Location: Situated in the Lake Agassiz Basin at the southern edge of Lake Manitoba in the Province of Manitoba, 22 km north of Portage la Prairie. Area: 23 000 ha. Wetland Type (Ramsar Classification System): Inland wetlands: Type 5 - permanent freshwater lakes; Type 8 - permanent freshwater ponds, marshes and swamps. Altitude: 248 m. Overview (Principal Characteristics): Delta Marsh consists of large basins and small sloughs connected to Lake Manitoba by several natural beaches. Physical Features (Geology, Geomorphology, Hydrology, Soils, Water, Climate): The area is subject to low level natural fluctuations caused by wind tides from Lake Manitoba. Average water depth is 1.2 m and maximum depth is 4 m. Ecological Features (Habitats, Vegetation): Vegetation consists of Phragmites communis and Scolochloa festucacea meadows which grade into wet prairie vegetation at slightly higher elevations. Sand ridges extending into the marsh contain considerable areas of mossy-cup oak Quercus macrocarpa. The beach ridge is dominated by box-elder Acer negundo and red alder Fraxinus pennsylvanica. Land Tenure: (a) Site: A combination of provincial Crown land and private land. (b) Surrounding Area: Private land. Conservation Measures Taken: About 16 600 ha are in public ownership as provincial Crown lands administered by the Wildlife Branch of the Manitoba Department of Natural Resources. -

Neotropical Migrant Banding at the Delta Marsh Bird Observatory, 1995
Neotropical migrant banding at the Delta Marsh Bird Observatory, 1995 Heidi A. den Haan Delta Marsh Bird Observatory Box 1, R.R. #1, Portage la Prairie, Manitoba R1N 3A1 Introduction consistent and accurate counts of birds during their migrations. It is part of a growing network of migration The Canadian boreal forest contains one of the most monitoring stations and is the only one in Manitoba. As diverse populations of breeding songbirds in North such, it plays a critical role in the collection of much America. Available evidence now suggests that several needed regional population data. Recent analyses species of neotropical migratory birds known to breed indicate that a long-term commitment is required to in this forest have been steadily decreasing, yet baseline obtain measurements of population changes that are information from this critical habitat in Canada is scarce amenable to useful interpretation. Therefore, a minimum at best. of five years of counts is likely to be needed to give any Birds are a good indicator of the health of the reliable indication of population trends; 10 years or more environment. Basic knowledge of population and are desirable (Hagan et al. 1994). DMBO is dedicated demographic changes in birds is needed to detect to serving this requirement into the future. declines, assess their importance, and provide a rational The Delta Marsh is one of North America’s largest basis for management decisions designed to ensure that marshes covering 21,870 hectares on the south end of populations are not allowed to decline to threatened or Lake Manitoba. Traditionally noted for it’s abundance endangered levels. -

Blue Jay, Vol.45, Issue 4
CANADA'S COMMITMENT TO THE "RAMSAR" CONVENTION BERT POSTON, Canadian Wildlife Service, Environment Canada, Room 230, 4999-98 Avenue, Edmonton, Alberta, T6B 2X3 and COLLEEN HYSLOP, Canadian Wildlife Ser¬ vice, Environment Canada, Ottawa, Ontario. K1A 0E7 Since references to "Ramsar" wetlands Bureau," consisting of an administrative are encountered periodically, an explana¬ unit based at IUCN Headquarters in tion of the term is needed. Ramsar is the Gland, Switzerland, and a unit to provide city in Iran in which in 1971 the "Con¬ technical and scientific advisory services vention on Wetlands of International Im¬ based at IWRB Headquarters in Slim- portance especially as Waterfowl Habitat" bridge, England. The Bureau carries out (also known as the Ramsar Convention) the work of the Parties, such as keeping was first drafted by 18 countries. By track of The List of wetlands and changes creating an international mechanism for to it, encouraging countries to join the the protection of wetlands, these countries Convention, arranging for studies to be demonstrated their commitment to the done on important wetland issues, and ar¬ conservation, management and wise use ranging for meetings of the Parties. of these environments, their fauna and flora. Canada acceded to the Ramsar Conven¬ tion in 1981 and designated Cap Since 1971 there have been three con¬ Tourmente National Wildlife Area, ferences of the "Parties to the Ramsar Con¬ Quebec, as Canada's first Ramsar wetland. vention" - in Cagliari, Italy (1980), in Fourteen more wetlands were added in Groningen, the Netherlands (1984) and in 1982, 2 in 1985, and 11 new sites were Regina, Canada from 27 May to 5 June announced at the 1987 conference for a 1987. -

The Loss of a Canadian Ornithological Treasure
Copyright © 2012 by the author(s). Published here under license by the Resilience Alliance. Hobson, K. A., D. R. Norris, G. Goldsborough, and S. G. Sealy. 2012. Requiem for a field station: the loss of a canadian ornithological treasure. Avian Conservation and Ecology 7(2): 7. http://dx.doi.org/10.5751/ ACE-00553-070207 Editorial Requiem for a field station: the loss of a Canadian ornithological treasure Requiem pour une station de recherche : perte d'un trésor ornithologique canadien Keith A. Hobson 1, D. Ryan Norris 2, Gordon Goldsborough 3 and Spencer G. Sealy 3 Key Words: Delta Marsh Bird Observatory; Delta Marsh Field Station; Lake Manitoba, Winnipeg Nestled on the southern shore of Lake Manitoba about 100 km We cannot do justice here to the many contributions made to northwest of Winnipeg (Fig. 1, 2), the University of Canadian ornithology by researchers working out of this Manitoba’s Delta Marsh Field Station (DMFS) had withstood station. Instead, and for the record, we list the ornithologically many tribulations since its creation by Dr. Jennifer M. Shay related theses and publications that were generated here OC in 1966. Many a financial and natural storm had done (Appendix 1, 2) and reflect on other contributions including battle with its relatively small group of academics, staff, the 18-year history of the Delta Marsh Bird Observatory that students, and friends who created it and supported a vision was hosted at the DMFS. This list is a minimum since there worthy of its potential over the years. And so, to some degree, are currently papers in review which we have not included when the flood waters lapped at its foundation in the spring here. -

Hydrology of the Delta Marsh Watershed: Water Balance Characterization and Analysis of Land Use Changes
Hydrology of the Delta Marsh Watershed: Water Balance Characterization and Analysis of Land Use Changes by Gregory John Schellenberg A thesis submitted to the Faculty of Graduate Studies of the University of Manitoba in partial fulfillment of the requirements for the degree of Master of Science Department of Civil Engineering University of Manitoba Winnipeg, Manitoba, Canada December 2017 Copyright © 2017 by Gregory John Schellenberg Abstract A hydrological model was used to examine the water balance of the Delta Marsh Wa- tershed (DMW) currently and as impacted by land use changes. Understanding DMW hydrology can help to improve conditions in the Delta Marsh. MIKE SHE model results showed that the water balance is typical of prairie conditions with limited wintertime activity, significant spring melt runoff, and high summertime evapotranspiration and infiltration. Results showed that the DMW contributes approximately 40 million m3 of water to the Delta Marsh in an average year, or 710 m3/ha/yr. Portage Creek is the single greatest inflow from the watershed (31% of total) and the West Marsh area also receives large runoff volumes (combined 37% of total). Analysis of land use changes showed that urban expansion in the DMW would increase annual marsh inflows by over 50% under one urbanization scenario due to associated decreases in infiltration and transpiration. An agricultural shift towards row crop predominance would have minimal impact on the DMW water balance. Conversion of cropland to natural vegetation would decrease annual runoff by 12% to the marsh due to increased surface ponding, infiltration, and transpiration. i Acknowledgments I would like to extend my sincerest gratitude to the following people/organizations, with- out whom this thesis would not be possible: My co-advsiors, Dr. -
Canadian Wetland Classification System
The Canadian Wetland Classification System Second Edition By the National Wetlands Working Group / Edited by B.G. Warner and C.D.A. Rubec 1997 by the Wetlands Research Centre, University of Waterloo, Waterloo, Ontario ISBN: 0-662-25857-6 Cat. No.: CW66-156/1997 Support for the production of this report was provided by the: • Canadian Wildlife Service, Environment Canada • Secretariat, North American Wetlands Conservation Council (Canada) • Wetlands Research Centre, University of Waterloo Copies of this report are available from: Wetlands Research Centre, Environmental Studies Building 1 University of Waterloo, Waterloo, Ontario N2L 3G1 Design: B. Winge, Graphics, University of Waterloo Cover photo credits: Top left: Ducks Unlimited (Canada) Top right: Doyle Wells (Natural Resources Canada) Bottom: B.G. Warner (Wetlands Research Centre) The Canadian Wetland Classification System Second Edition By the National Wetlands Working Group / Edited by B.G. Warner and C.D.A. Rubec Stephen Zoltai 1928-1997 This second edition of The Canadian Wetland Classification System is dedicated to our colleague, Stephen Zoltai for his contribution to Canadian wetlands science and his encouragement to all of the members of the National Wetlands Working Group to complete this volunteer ten-year task. It was a privilege to know and work with this remarkable man. Contributors Glen Adams Canadian Wildlife Service, Environment Canada, Saskatoon, Saskatchewan Pierre Buteau Ministère des Ressources naturelles du Québec, Sainte-Foy, Québec Norman Dignard Ministère -
English, French, Spanish)
NATIONAL REPORT ON THE IMPLEMENTATION OF THE RAMSAR CONVENTION ON WETLANDS National Reports to be submitted to the 11th Meeting of the Conference of the Contracting Parties, Romania, June 2012 Please submit the completed National Report, in electronic (Microsoft Word) format, and preferably by e-mail, to the Ramsar Secretariat by 15 September 2011. National Reports should be sent to: Alexia Dufour, Regional Affairs Officer, Ramsar Secretariat ([email protected]) National Report Format for Ramsar COP11, page 2 Introduction & background 1. This National Report Format (NRF) has been approved by the Standing Committee in Decision SC41-24 for the Ramsar Convention’s Contracting Parties to complete as their national reporting to the 11th meeting of the Conference of the Contracting Parties of the Convention (Bucharest, Romania, June 2012). 2. Following Standing Committee discussions at its 40th meeting in May 2009, and its Decision SC40-29, this COP11 National Report Format closely follows that used for the COP10 National Report Format, which in turn was a significantly revised and simplified format in comparison with the National Report Formats provided to previous recent COPs. 3. In addition to thus permitting continuity of reporting and implementation progress analyses by ensuring that indicator questions are as far as possible consistent with previous NRFs (and especially the COP10 NRF), this COP11 NRF is structured in terms of the Goals and Strategies of the 2009-2015 Ramsar Strategic Plan adopted at COP10 as Resolution X.1, and the indicators