Facilitators and Barriers to Safely Managed Water and Sanitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table S1 the Detailed Information of Garlic Samples Table S2 Sensory
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2019 Table S1 The detailed information of garlic samples NO. Code Origin Cultivar 1 SD1 Lv County, Rizhao City, Shandong Rizhaohong 2 SD2 Jinxiang County, Jining City, Shandong Jinxiang 3 SD3 Chengwu County, Heze City, Shandong Chengwu 4 SD4 Lanshan County, Linyi City, Shandong Ershuizao 5 SD5 Anqiu City, Weifang City, Shandong Anqiu 6 SD6 Lanling County, Linyi City, Shandong Cangshan 7 SD7 Laicheng County, Laiwu City, Shandong Laiwu 8 JS1 Feng County, Xuzhou City, Jiangsu Taikongerhao 9 JS2 Pei County, Xuzhou City, Jiangsu Sanyuehuang 10 JS3 Tongshan County, Xuzhou City, Jiangsu Lunong 11 JS4 Jiawang County, Xuzhou City, Jiangsu Taikongzao 12 JS5 Xinyi County, Xuzhou City, Jiangsu Yandu 13 JS6 Pizhou County, Xuzhou City, Jiangsu Pizhou 14 JS7 Quanshan County, Xuzhou City, Jiangsu erjizao 15 HN1 Zhongmou County, Zhengzhou City, Sumu 16 HN2 Huiji County, ZhengzhouHenan City, Henan Caijiapo 17 HN3 Lankao County, Kaifeng City, Henan Songcheng 18 HN4 Tongxu County, Kaifeng City, Henan Tongxu 19 HN5 Weishi County, Kaifeng City, Henan Liubanhong 20 HN6 Qi County, Kaifeng City, Henan Qixian 21 HN7 Minquan County, Shangqiu City, Henan Minquan 22 YN1 Guandu County, Kunming City, Yunnan Siliuban 23 YN2 Mengzi County, Honghe City, Yunnan Hongqixing 24 YN3 Chenggong County, Kunming City, Chenggong 25 YN4 Luliang County,Yunnan Qujing City, Yunnan Luliang 26 YN5 Midu County, Dali City, Yunnan Midu 27 YN6 Eryuan County, Dali City, Yunnan Dali 28 -

Produzent Adresse Land Allplast Bangladesh Ltd
Zeitraum - Produzenten mit einem Liefertermin zwischen 01.01.2020 und 31.12.2020 Produzent Adresse Land Allplast Bangladesh Ltd. Mulgaon, Kaliganj, Gazipur, Rfl Industrial Park Rip, Mulgaon, Sandanpara, Kaligonj, Gazipur, Dhaka Bangladesh Bengal Plastics Ltd. (Unit - 3) Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh HKD International (Cepz) Ltd. Plot # 49-52, Sector # 8, Cepz, Chittagong Bangladesh Lhotse (Bd) Ltd. Plot No. 60 & 61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Plastoflex Doo Branilaca Grada Bb, Gračanica, Federacija Bosne I H Bosnia-Herz. ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Konrrg Pisey, Kampong Spueu Cambodia Powerjet Home Product (Cambodia) Co., Ltd. Manhattan (Svay Rieng) Special Economic Zone, National Road 1, Sangkat Bavet, Krong Bavet, Svaay Rieng Cambodia AJS Electronics Ltd. 1st Floor, No. 3 Road 4, Dawei, Xinqiao, Xinqiao Community, Xinqiao Street, Baoan District, Shenzhen, Guangdong China AP Group (China) Co., Ltd. Ap Industry Garden, Quetang East District, Jinjiang, Fujian China Ability Technology (Dong Guan) Co., Ltd. Songbai Road East, Huanan Industrial Area, Liaobu Town, Donggguan, Guangdong China Anhui Goldmen Industry & Trading Co., Ltd. A-14, Zongyang Industrial Park, Tongling, Anhui China Aold Electronic Ltd. Near The Dahou Viaduct, Tianxin Industrial District, Dahou Village, Xiegang Town, Dongguan, Guangdong China Aurolite Electrical (Panyu Guangzhou) Ltd. Jinsheng Road No. 1, Jinhu Industrial Zone, Hualong, Panyu District, Guangzhou, Guangdong China Avita (Wujiang) Co., Ltd. No. 858, Jiaotong Road, Wujiang Economic Development Zone, Suzhou, Jiangsu China Bada Mechanical & Electrical Co., Ltd. No. 8 Yumeng Road, Ruian Economic Development Zone, Ruian, Zhejiang China Betec Group Ltd. -

SGS-Safeguards 04910- Minimum Wages Increased in Jiangsu -EN-10
SAFEGUARDS SGS CONSUMER TESTING SERVICES CORPORATE SOCIAL RESPONSIILITY SOLUTIONS NO. 049/10 MARCH 2010 MINIMUM WAGES INCREASED IN JIANGSU Jiangsu becomes the first province to raise minimum wages in China in 2010, with an average increase of over 12% effective from 1 February 2010. Since 2008, many local governments have deferred the plan of adjusting minimum wages due to the financial crisis. As economic results are improving, the government of Jiangsu Province has decided to raise the minimum wages. On January 23, 2010, the Department of Human Resources and Social Security of Jiangsu Province declared that the minimum wages in Jiangsu Province would be increased from February 1, 2010 according to Interim Provisions on Minimum Wages of Enterprises in Jiangsu Province and Minimum Wages Standard issued by the central government. Adjustment of minimum wages in Jiangsu Province The minimum wages do not include: Adjusted minimum wages: • Overtime payment; • Monthly minimum wages: • Allowances given for the Areas under the first category (please refer to the table on next page): middle shift, night shift, and 960 yuan/month; work in particular environments Areas under the second category: 790 yuan/month; such as high or low Areas under the third category: 670 yuan/month temperature, underground • Hourly minimum wages: operations, toxicity and other Areas under the first category: 7.8 yuan/hour; potentially harmful Areas under the second category: 6.4 yuan/hour; environments; Areas under the third category: 5.4 yuan/hour. • The welfare prescribed in the laws and regulations. CORPORATE SOCIAL RESPONSIILITY SOLUTIONS NO. 049/10 MARCH 2010 P.2 Hourly minimum wages are calculated on the basis of the announced monthly minimum wages, taking into account: • The basic pension insurance premiums and the basic medical insurance premiums that shall be paid by the employers. -

Climate Research 57:123
Vol. 57: 123–132, 2013 CLIMATE RESEARCH Published August 20 doi: 10.3354/cr01174 Clim Res Extreme sea ice events in the Chinese marginal seas during the past 2000 years Jie Fei1,2,*, Zhong-Ping Lai2,3, David D. Zhang4, Hong-Ming He5 1Institute of Chinese Historical Geography, Fudan University, Shanghai 200433, PR China 2State Key Laboratory of Cryospheric Sciences (CAREERI), Chinese Academy of Sciences, Lanzhou 730000, PR China 3Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, PR China 4Department of Geography, The University of Hong Kong, Hong Kong SAR 5Institute of Soil and Water Conservation, Chinese Academy of Sciences & Ministry of Water Resources, Yangling District 712100, PR China ABSTRACT: We used Chinese historical literature to examine extreme records of sea ice events in the Chinese marginal seas during the past 2000 yr. We identified a total of 6 sea ice events that occurred in the sea areas to the south of 35° N. These extreme events occurred in the winters of AD 821/822, 903/904, 1453/1454, 1493/1494, 1654/1655 and 1670/1671. According to the histori- cal records, the southern limit of sea ice in the Chinese marginal seas should be in Hangzhou Bay (30−31° N), and most probably near Haiyan County in Zhejiang Province (30.5° N). The sea ice events of 1453/1454 and 1654/1655 were synchronous with the freezing events of Taihu Lake, and the sea ice event of 1670/1671 was synchronous with the freezing event of the lower reaches of the Yangtze River. However, none of the sea ice events was synchronous with the abnormally early freezing dates of Suwa Lake in Japan or the extreme freezing events of Venice Lagoon in Italy. -
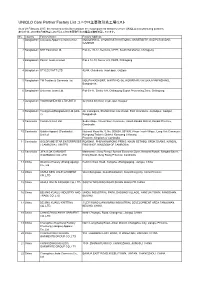
UNIQLO Core Partner Factory List ユニクロ主要取引先工場リスト
UNIQLO Core Partner Factory List ユニクロ主要取引先工場リスト As of 28 February 2017, the factories in this list constitute the major garment factories of core UNIQLO manufacturing partners. 本リストは、2017年2月末時点におけるユニクロ主要取引先の縫製工場を掲載しています。 No. Country Factory Name Factory Address 1 Bangladesh Colossus Apparel Limited unit 2 MOGORKHAL, CHOWRASTA NATIONAL UNIVERSITY, GAZIPUR SADAR, GAZIPUR 2 Bangladesh NHT Fashions Ltd. Plot no. 20-22, Sector-5, CEPZ, South Halishahar, Chittagong 3 Bangladesh Pacific Jeans Limited Plot # 14-19, Sector # 5, CEPZ, Chittagong 4 Bangladesh STYLECRAFT LTD 42/44, Chandona, Joydebpur, Gazipur 5 Bangladesh TM Textiles & Garments Ltd. MOUZA-KASHORE, WARD NO.-06, HOBIRBARI,VALUKA,MYMENSHING, Bangladesh. 6 Bangladesh Universal Jeans Ltd. Plot 09-11, Sector 6/A, Chittagong Export Processing Zone, Chittagong 7 Bangladesh YOUNGONES BD LTD UNIT-II 42 (3rd & 4th floor) Joydevpur, Gazipur 8 Bangladesh Youngones(Bangladesh) Ltd.(Unit- 24, Laxmipura, Shohid chan mia sharak, East Chandona, Joydebpur, Gazipur, 2) Bangladesh 9 Cambodia Cambo Unisoll Ltd. Seda village, Vihear Sour Commune, Ksach Kandal District, Kandal Province, Cambodia 10 Cambodia Golden Apparel (Cambodia) National Road No. 5, No. 005634, 001895, Phsar Trach Village, Long Vek Commune, Limited Kompong Tralarch District, Kompong Chhnang Province, Kingdom of Cambodia. 11 Cambodia GOLDFAME STAR ENTERPRISES ROAD#21, PHUM KAMPONG PRING, KHUM SETHBO, SROK SAANG, KANDAL ( CAMBODIA ) LIMITED PROVINCE, KINGDOM OF CAMBODIA 12 Cambodia JIFA S.OK GARMENT Manhattan ( Svay Rieng ) Special Economic Zone, National Road#, Sangkat Bavet, (CAMBODIA) CO.,LTD Krong Bavet, Svay Rieng Province, Cambodia 13 China Okamoto Hosiery (Zhangjiagang) Renmin West Road, Yangshe, Zhangjiagang, Jiangsu, China Co., Ltd 14 China ANHUI NEW JIALE GARMENT WenChangtown, XuanZhouDistrict, XuanCheng City, Anhui Province CO.,LTD 15 China ANHUI XINLIN FASHION CO.,LTD. -

GU 03032021 SHM 02 Nonfo
Produzent Adresse Land A.M. Design Ltd. Diakhali, Baron, Ashulia, Savar, Dhaka Bangladesh AB Apparels Ltd. 225, Singair Road, Tetuljhora, Hemayetpur, Savar, Dhaka Bangladesh ASR Sweater Ltd. Mulaid, Mawna, Sreepur, Gazipur, Dhaka Bangladesh Abanti Colour Tex Ltd. Plot-M-A-646, Shashongaon, Enayetnagar, Fatullah, Narayanganj, Dhaka Bangladesh Ador Composite Ltd. 1, C; B Bazar, Gilarchala, Sreepur, Gazipur, Bd Gazipur District, Gazipur, Dhaka Bangladesh Advanced Composite Textile Ltd. Kashor Masterbari, Bhaluka, Mymensingh, Sylhet Bangladesh Ahmed Fashions 34/1, Darus Salam Road, Dhaka Bangladesh Alim Knit (Bd) Ltd. Kashimpur, Nayapara, Gazipur, Gazipur, Dhaka Bangladesh Antim Knit Composite Ltd. Barpa, Rupshi, Rupgonj, Narayangonj, Dhaka Bangladesh Antim Knitting Dyeing & Finishing Ltd. Barpa, Rupshi, Rupgonj, Narayangonj, Dhaka Bangladesh Apparel Promoters Ltd. 1206/A, Nasirabad I/A, Bayzid Thana Road, Bayzid, Chittagong Bangladesh Aspire Garments Ltd. 491 Dhalla, Singair, Manikganj, Dhaka Bangladesh BHML Industries Ltd. Kamarjuri, Natun Bazar, National University, Gazipur, Dhaka Bangladesh BKC Sweater, Ltd. Plot No. 212-214, Dagerchala Main Road, Dagerchala, National University, Gazipur, Dhaka Bangladesh Basic Apparels Ltd. 135-138, Abdullahpur, Uttara, Dhaka Bangladesh Birds A & Z Ltd. Plot No. 113, Baipail, Savar, Dhaka Bangladesh Blue Planet Fashionwear Ltd. Kewa, Sreepur, Gazipur, Dhaka Bangladesh Bottoms Gallery (Pvt.) Ltd. Bulbul Tower, Dighirchala, Chandona, Joydebpur, Gazipur, Dhaka Bangladesh Chaity Composite Ltd. Chotto Silmondi, Tripurdi, Sonargaon, Narayangonj, Dhaka Bangladesh Crony Tex Sweater Ltd. Black B, Bscic Industrial Estate, Narajangonj, Dhaka Bangladesh Crown Exclusive Wears Ltd. Mawna, Sreepur, Gazipur, Dhaka Bangladesh Crown Fashion & Sweater Ind. Ltd. Plot. 781-782, Vogra, Joydebpur, P.O. Vogra, P.S. Joydebpur, Dist. Gazipur, Gazipur, Dhaka Bangladesh Crown Knitwear Ltd. 781/782, Vogra, Chourasta, Gazipur, Dhaka Bangladesh Deluxe Fashions Ltd. -
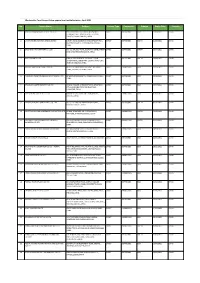
Apparel & Textile Factory List
Woolworths Food Group-Active apparel and textile factories- April 2021 No. Factory Name Address Factory Type Commodity Scheme Expiry Date Country 1 JIANGSU HONGMOFANG TEXTILE CO., LTD EAST CIFU ROAD, EAST RUISHENG AVENUE,, DIRECT SOFTGOODS BSCI 23/04/2021 CHINA ECONOMIC DEVELOPMENT ZONE, SHUYANG COUNTY,,SUQIAN,JIANGSU,,CHINA 2 LIANYUNGANG ZHAOWEN SHOES LIMITED 2 BEIHAI ROAD,ECONOMIC DEVELOPMENT AREA, DIRECT SOFTGOODS SMETA 11/05/2021 CHINA GUANNAN COUNTY,,LIANYUNGANG,JIANGSU,, CHINA 3 ZHUCHENG YIXIN GARMENT CO., LTD NO. 321 SHUNDU ROAD,,ECONOMIC DEVELOPMENT DIRECT SOFTGOODS SMETA 13/07/2021 CHINA ZONE,,ZHUCHENG,SHANDONG,,CHINA 4 HRX FASHION CO LTD 1000 METERS SOUTH OF GUOCANG TOWN DIRECT SOFTGOODS SMETA 25/08/2021 CHINA GOVERNMENT,,WENSHANG COUNTY, JINING CITY, JINING,SHANDONG,,CHINA 5 PUJIANG KINGSHOW CARPET CO LTD NO.75-1 ZHEN PU ROAD, PU JIANG, ZHE JIANG, DIRECT HARDGOODS BSCI 24/12/2021 CHINA CHINA,,PUJIANG,ZHEJIANG,,CHINA 6 YANGZHOU TENGYI SHOES MANUFACTURE CO., LTD. 13 WEST HONGCHENG RD,,,FANGXIANG,JIANGSU,, DIRECT SOFTGOODS BSCI 22/10/2021 CHINA CHINA 7 ZHAOYUAN CASTTE GARMENT CO LTD PANJIAJI VILLAGE, LINGLONG TOWN,,ZHAOYUAN DIRECT SOFTGOODS BSCI 06/07/2021 CHINA CITY, SHANDONG PROVINCE,ZHAOYUAN, SHANDONG,,CHINA 8 RUGAO HONGTAI TEXTILE CO LTD XINJIAN VILLAGE, JIANGAN TOWN,,RUGAO, DIRECT HARDGOODS BSCI 07/12/2021 CHINA JIANGSU,,CHINA 9 NANJING BIAOMEI HOMETEXTILES CO.,LTD NO.13 ERST WUCHU ROAD,HENGXI TOWN,,, DIRECT SOFTGOODS SMETA 04/11/2021 CHINA NANJING,JIANGSU,,CHINA 10 NANTONG YAOXING HOUSEWARE PRODUCTS CO.,LTD NO.999, TONGFUBEI RD., CHONGCHUAN, DIRECT HARDGOODS SMETA 24/09/2021 CHINA NANTONG,,NANTONG,JIANGSU,,CHINA 11 CHAOZHOU CHAOAN ZHENGYUN CERAMICS QIAO HU VILLAGE, CHAOAN, CHAOZHOU CITY, DIRECT HARDGOODS SMETA 19/05/2021 CHINA INDUSTRIAL CO LTD GUANGDONG, CHINA,,CHAOZHOU,GUANGDONG,, CHINA 12 YANTAI PACIFIC HOME FASHION FUSHAN MILL NO. -

Climate Research 50:161
Vol. 50: 161–170, 2011 CLIMATE RESEARCH Published December 22 doi: 10.3354/cr01052 Clim Res Contribution to CR Special 28 ‘Changes in climatic extremes over mainland China’ OPENPEN ACCESSCCESS Historical analogues of the 2008 extreme snow event over Central and Southern China Zhixin Hao1, Jingyun Zheng1, Quansheng Ge1,*, Wei-Chyung Wang2 1Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, 11A Datun Road, Chaoyang District, Beijing 100101, PR China 2Atmospheric Sciences Research Center, State University of New York, Albany, New York 12203, USA ABSTRACT: We used weather records contained in Chinese historical documents from the past 500 yr to search for extreme snow events (ESEs) that were comparable in severity to an event in early 2008, when Central and Southern China experienced persistent heavy snowfall with unusu- ally low temperatures. ESEs can be divided into 3 groups according to the geographical coverage of snowfall, using the following criteria to define an ESE: >15 snowfall days, 20 snow-cover/icing days, and 30 cm total cumulated snow depth for an individual winter. The first group covers the whole of Eastern China (East of 105° E), and ESEs occurred in 1654, 1660, 1665, 1670, 1676, 1683, 1689, 1690, 1700, 1714, 1719, 1830−32, 1840, 1877 and 1892; the second group is located mainly in the area south of Huaihe River (~33° N), and ESEs occured in 1694, 1887, 1929, and 1930; and the third group is confined within the central region between Yellow River and Nanling Mountain (roughly 26° to 35° N), and ESEs occurred in 1578, 1620, 1796, and 1841. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Addition of Clopidogrel to Aspirin in 45 852 Patients with Acute Myocardial Infarction: Randomised Placebo-Controlled Trial
Articles Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group* Summary Background Despite improvements in the emergency treatment of myocardial infarction (MI), early mortality and Lancet 2005; 366: 1607–21 morbidity remain high. The antiplatelet agent clopidogrel adds to the benefit of aspirin in acute coronary See Comment page 1587 syndromes without ST-segment elevation, but its effects in patients with ST-elevation MI were unclear. *Collaborators and participating hospitals listed at end of paper Methods 45 852 patients admitted to 1250 hospitals within 24 h of suspected acute MI onset were randomly Correspondence to: allocated clopidogrel 75 mg daily (n=22 961) or matching placebo (n=22 891) in addition to aspirin 162 mg daily. Dr Zhengming Chen, Clinical Trial 93% had ST-segment elevation or bundle branch block, and 7% had ST-segment depression. Treatment was to Service Unit and Epidemiological Studies Unit (CTSU), Richard Doll continue until discharge or up to 4 weeks in hospital (mean 15 days in survivors) and 93% of patients completed Building, Old Road Campus, it. The two prespecified co-primary outcomes were: (1) the composite of death, reinfarction, or stroke; and Oxford OX3 7LF, UK (2) death from any cause during the scheduled treatment period. Comparisons were by intention to treat, and [email protected] used the log-rank method. This trial is registered with ClinicalTrials.gov, number NCT00222573. or Dr Lixin Jiang, Fuwai Hospital, Findings Allocation to clopidogrel produced a highly significant 9% (95% CI 3–14) proportional reduction in death, Beijing 100037, P R China [email protected] reinfarction, or stroke (2121 [9·2%] clopidogrel vs 2310 [10·1%] placebo; p=0·002), corresponding to nine (SE 3) fewer events per 1000 patients treated for about 2 weeks. -

Results Announcement for the Year Ended December 31, 2020
(GDR under the symbol "HTSC") RESULTS ANNOUNCEMENT FOR THE YEAR ENDED DECEMBER 31, 2020 The Board of Huatai Securities Co., Ltd. (the "Company") hereby announces the audited results of the Company and its subsidiaries for the year ended December 31, 2020. This announcement contains the full text of the annual results announcement of the Company for 2020. PUBLICATION OF THE ANNUAL RESULTS ANNOUNCEMENT AND THE ANNUAL REPORT This results announcement of the Company will be available on the website of London Stock Exchange (www.londonstockexchange.com), the website of National Storage Mechanism (data.fca.org.uk/#/nsm/nationalstoragemechanism), and the website of the Company (www.htsc.com.cn), respectively. The annual report of the Company for 2020 will be available on the website of London Stock Exchange (www.londonstockexchange.com), the website of the National Storage Mechanism (data.fca.org.uk/#/nsm/nationalstoragemechanism) and the website of the Company in due course on or before April 30, 2021. DEFINITIONS Unless the context otherwise requires, capitalized terms used in this announcement shall have the same meanings as those defined in the section headed “Definitions” in the annual report of the Company for 2020 as set out in this announcement. By order of the Board Zhang Hui Joint Company Secretary Jiangsu, the PRC, March 23, 2021 CONTENTS Important Notice ........................................................... 3 Definitions ............................................................... 6 CEO’s Letter .............................................................. 11 Company Profile ........................................................... 15 Summary of the Company’s Business ........................................... 27 Management Discussion and Analysis and Report of the Board ....................... 40 Major Events.............................................................. 112 Changes in Ordinary Shares and Shareholders .................................... 149 Directors, Supervisors, Senior Management and Staff.............................. -

Factory Address Country
Factory Address Country Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh Lhotse (BD) Ltd. Plot No. 60&61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Bengal Plastics Ltd. Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Korng Pisey District, Konrrg Pisey, Kampong Speu Cambodia Ningbo Zhongyuan Alljoy Fishing Tackle Co., Ltd. No. 416 Binhai Road, Hangzhou Bay New Zone, Ningbo, Zhejiang China Ningbo Energy Power Tools Co., Ltd. No. 50 Dongbei Road, Dongqiao Industrial Zone, Haishu District, Ningbo, Zhejiang China Junhe Pumps Holding Co., Ltd. Wanzhong Villiage, Jishigang Town, Haishu District, Ningbo, Zhejiang China Skybest Electric Appliance (Suzhou) Co., Ltd. No. 18 Hua Hong Street, Suzhou Industrial Park, Suzhou, Jiangsu China Zhejiang Safun Industrial Co., Ltd. No. 7 Mingyuannan Road, Economic Development Zone, Yongkang, Zhejiang China Zhejiang Dingxin Arts&Crafts Co., Ltd. No. 21 Linxian Road, Baishuiyang Town, Linhai, Zhejiang China Zhejiang Natural Outdoor Goods Inc. Xiacao Village, Pingqiao Town, Tiantai County, Taizhou, Zhejiang China Guangdong Xinbao Electrical Appliances Holdings Co., Ltd. South Zhenghe Road, Leliu Town, Shunde District, Foshan, Guangdong China Yangzhou Juli Sports Articles Co., Ltd. Fudong Village, Xiaoji Town, Jiangdu District, Yangzhou, Jiangsu China Eyarn Lighting Ltd. Yaying Gang, Shixi Village, Shishan Town, Nanhai District, Foshan, Guangdong China Lipan Gift & Lighting Co., Ltd. No. 2 Guliao Road 3, Science Industrial Zone, Tangxia Town, Dongguan, Guangdong China Zhan Jiang Kang Nian Rubber Product Co., Ltd. No. 85 Middle Shen Chuan Road, Zhanjiang, Guangdong China Ansen Electronics Co. Ning Tau Administrative District, Qiao Tau Zhen, Dongguan, Guangdong China Changshu Tongrun Auto Accessory Co., Ltd.