Size−Shape Relationships in the Mesozoic Planispiral Ammonites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post-Carboniferous Stratigraphy, Northeastern Alaska by R
Post-Carboniferous Stratigraphy, Northeastern Alaska By R. L. DETTERMAN, H. N. REISER, W. P. BROSGE,and]. T. DUTRO,JR. GEOLOGICAL SURVEY PROFESSIONAL PAPER 886 Sedirnentary rocks of Permian to Quaternary age are named, described, and correlated with standard stratigraphic sequences UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON 1975 UNITED STATES DEPARTMENT OF THE INTERIOR ROGERS C. B. MORTON, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress Cataloging in Publication Data Detterman, Robert L. Post-Carboniferous stratigraphy, northeastern Alaska. (Geological Survey Professional Paper 886) Bibliography: p. 45-46. Supt. of Docs. No.: I 19.16:886 1. Geology-Alaska. I. Detterman, Robert L. II. Series: United States. Geological Survey. Professional Paper 886. QE84.N74P67 551.7'6'09798 74-28084 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-02687-2 CONTENTS Page Page Abstract __ _ _ _ _ __ __ _ _ _ _ _ _ _ _ _ _ _ _ __ __ _ _ _ _ _ _ __ __ _ _ __ __ __ _ _ _ _ __ 1 Stratigraphy__:_Continued Introduction __________ ----------____ ----------------____ __ 1 Kingak Shale ---------------------------------------- 18 Purpose and scope ----------------------~------------- 1 Ignek Formation (abandoned) -------------------------- 20 Geographic setting ------------------------------------ 1 Okpikruak Formation (geographically restricted) ________ 21 Previous work and acknowledgments ------------------ 1 Kongakut Formation ---------------------------------- -

Stratigraphic Implications of a New Lower Cretaceous Ammonoid Fauna from the Puez Area (Valanginian – Aptian, Dolomites, Southern Alps, Italy)
Geo.Alp, Vol. 3, S. 55–83, 2006 STRATIGRAPHIC IMPLICATIONS OF A NEW LOWER CRETACEOUS AMMONOID FAUNA FROM THE PUEZ AREA (VALANGINIAN – APTIAN, DOLOMITES, SOUTHERN ALPS, ITALY) Alexander Lukeneder1 & Christian Aspmair2 With 6 figures and 8 plates 1 Natural History Museum, Geological-Palaeontological Department, Burgring 7, A-1010 Wien, Austria, e-mail: [email protected] 2 Prissian 102, I – 39010 Tisens (BZ), Italy Abstract Lower Cretaceous ammonoids (n = 424) were collected at the Puez locality in the Dolomites of Southern Tyrol. The cephalopod fauna from the marly limestones to marls here indicates Late Valanginian to Early Aptian age. The deposition of the marly limestones and marls of this interval occurred during depositional- ly unstable conditions. The underlying Biancone Formation (Maiolica Formation) is of Early Valanginian, whereas the lowermost Rosso Ammonitico is of Jurassic to Berriasian age. The ammonoid fauna consists of 27 different genera, each represented by 1-2 species. The assemblage at the Puez section is dominated by the Phylloceratina (30%) and the Ammonitina (34%). Phyllopachyceras (17%) and Phylloceras (13%) (both Phylloceratina) are the most frequent components, followed by Lytoceras (12%) (Lytoceratina), and Barremites (10%) and Melchiorites (8%) (both Ammonitina). The cephalopod fauna is purely of Mediterranean origin. Zusammenfassung Unterkreide Ammonoideen (424 Exemplare) der Puez Lokalität in den Dolomiten Süd-Tirols wurden unter- sucht. Die Fauna der mergeligen Kalke und Mergel von Puez zeigen ein Alter von Ober-Valanginium bis Unter-Aptium an. Die mergeligen Kalke und Mergel dieses Abschnitts lagerten sich unter instabiler Bedingungen ab. Die unterlagernde Biancone Formation (Maiolica Formation) zeigt Unter-Valanginium an, wogegen die tiefste Formation des Rosso Ammonitico auf Ober-Jura bis Berriasium hindeutet. -

Stratigraphy and General Geology of Jke Mccarthy C-5 Quadrangle; Alaska
Stratigraphy and General Geology of jke McCarthy C-5 Quadrangle; Alaska By E. M. MAcKEVETT, JR. Descriptions of the rocks of a quadrangle famous for its copper mines UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1971 UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. 79-609946 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 30 cents (paper cover) CONTENTS Page Abstract . .....-.. .... ........_.. .. .. .. .. .. .. 1 Introduction ...-. ..... .... .... .. .. ... .. ._. 1 Geology -. ... _. __ .._____ _ . _ __.. _ __. ____. 4 General summary, regional setting, and structure......................... __...... 4 Stratigraphy and lithology . ... __. _ . 8 Hasen Creek Formation .. .. _ . ._.. 8 Nikolai Greenstone ..__ ......_ . _ 8 Chitistone Limestone ... .... ._ . __ _... _ _ _ . 10 Nizina Limestone . .... _ .. .._._ _..._ . _. 11 McCarthy Formation ._ . ... 13 Lower member ...... _...__. __ _ . ._ . _ ._ 13 Upper member . _ .. __. _ .. 14 Lubbe Creek Formation _. __ .. 15 Nizina Mountain Formation .. _ . _-- . .. 16 Root Glacier Formation . __. ._.. ..__ . .. 17 Kennicott Formation .. .__. _. ___ ___. _ ' ._ 19 Schulze Formation ..._... ___ __.. 20 Moonshine Creek Formation _.. ... _ 20 Frederika Formation .. _. .. _. _. 21 Intrusive rocks . .. _. .._... .. 23 Andesitic dikes and sills. ......................... ,.._ . .................. 23 Intermediate rocks ......................................-.......-..^.....................-. -

Aptian Ammonites from the Argentinian Austral Basin
ANNALS OF THE SOUTH AFRICAN MUSEUM ANN ALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 96 Band December 1986 Desember Part 7 Deel APTIAN AMMONITES FROM THE ARGENTINIAN AUSTRAL BASIN. THE SUBFAMILY HELICANCYLINAE HYATT, 1894 By MARIA BEATRIZ AGUIRRE URRETA Cape Town Kaapstad APTIAN AMMONITES FROM THE ARGENTINIAN AUSTRAL BASIN. THE SUBFAMILY HELICANCYLINAE HYATT, 1894 By MARIA BEATRIZ AGUIRRE URRETA Department of Invertebrate Palaeontology, South African Museum, Cape Town* (With 19 figures) [MS accepted I October 1984] ABSTRACT Representatives of the subfamily Helicancylinae are locally common in deposits of Aptian age in the northern central Austral Basin, Patagonia. A strati graphical synthesis of the Lower Cretaceous deposits in the area studied is outlined. Schematic sequences of the measured sections at the principal localities, which also exhibit the various levels containing ammonites, are shown. The section on systematic palaeontology comprises a discussion of the subfamily Helicancylinae, and generic and specific descriptions of all taxa represented in the Austral Basin. In addition to the study of the Patagonian material, bibliographical research reveals the necessity of redefining the genera Helicancylus and Hamiticeras in order to clarify the systematics of the subfamily. The following species are identified: Helicancylus patagonicus, Helicancylus bonarellii, Toxo ceratoides nagerai, Toxoceratoides cf. biplex, Toxoceratoides? haughtoni, Toxoceratoides? sp., and Tonohamites aequicingulatus. The fauna shows some affinities with that of Zululand and western Europe. CONTENTS PAGE Introduction. .. 271 Location of specimens . 273 Dimensions . 273 Stratigraphic synthesis . 273 History of palaeontological research . 279 Systematic palaeontology ............................... 280 Concluding remarks. 311 Acknowledgements. 311 References. 312 INTRODUCTION The subfamily Helicancylinae comprises a group of small ancyloceratids that have a nearly worldwide distribution. -

Geological Survey Canada
1-32 GEOLOGICAL PAPER 70-32 SURVEY OF CANADA DEPARTMENT OF ENERGY, MINES AND RESOURCES BROCK RIVER MAP-AREA, DISTRICT OF MACKENZIE (97 D) (Report, 6 figures, 2 tables and P.S. Map 13-1970) H. R. Balkwill and C. J. Yorath Price, $2.00 1970 GEOLOGICAL SURVEY OF CANADA CANADA PAPER 70-32 BROCK RIVER MAP-AREA, DISTRICT OF MACKENZIE (97 D) H. R. Balkwill and C. J. Yorath DEPARTMENT OF ENERGY, MINES AND RESOURCES @)Crown Copyrights reserved Available by mail from Information Canada, Ottawa from the Geological Survey of Canada 601 Booth St., Ottawa and Information Canada bookshops in HALIFAX - 1735 Barrington Street MONTREAL - 1182 St. Catherine Street West OTTAWA - 171 Slater Street TORONTO - 221 Yonge Street WINNIPEG - 499 Portage Avenue VANCOUVER - 657 Granville Street or through your bookseller Price: $2.00 Catalogue No. M:44-70-32 Price subject to change without notice Information Canada Ottawa 1971 - iii - CONTENTS Page Abstract.............................. ...... ................ ... ....... v Introduction . 1 Physiography . 1 Stratigraphy . 5 Proterozoic.............. 8 Shaler Group . • . 8 Diabase sills and dykes . 11 Age and correlation of Proterozoic rocks . 11 Paleozoic . 12 Old Fort Island Formation . 12 Mount Cap Formation . .. 13 Saline River Formation . 15 'Ronning Group' . 15 Bear Rock Formation . 16 Cretaceous . 17 •Silty zone' . 18 1Benton°itic zone' . 18 Age and correlation of Cretaceous rocks . 18 Quaternary . 19 Structural Geology...... 19 Coppermine Arch . 19 Horton Plain and Wollaston structural basin . 20 Structural control of topography . 21 Economic Geology ..................... ........................ ·. 21 Addendum . 22 References 23 Illustrations Map 13- 1970: Geology, Brock River area (97D), District of Mackenzie ..... in pocket Table 1. Table of map-units . -

A Review of the Classification of Jurassic Aspidoceratid Ammonites – the Superfamily Aspidoceratoidea
VOLUMINA JURASSICA, 2020, XVIII (1): 47–52 DOI: 10.7306/VJ.18.4 A review of the classification of Jurassic aspidoceratid ammonites – the Superfamily Aspidoceratoidea Horacio PARENT1, Günter SCHWEIGERT2, Armin SCHERZINGER3 Key words: Superfamily Aspidoceratoidea, Aspidoceratidae, Epipeltoceratinae emended, Peltoceratidae, Gregoryceratinae nov. subfam. Abstract. The aspidoceratid ammonites have been traditionally included in the superfamily Perisphinctoidea. However, the basis of this is unclear for they bear unique combinations of characters unknown in typical perisphinctoids: (1) the distinct laevaptychus, (2) stout shells with high growth rate of the whorl section area, (3) prominent ornamentation with tubercles, spines and strong growth lines running in parallel over strong ribs, (4) lack of constrictions, (5) short to very short bodychamber, and (6) sexual dimorphism characterized by minia- turized microconchs and small-sized macroconchs besides the larger ones, including changes of sex during ontogeny in many cases. Considering the uniqueness of these characters we propose herein to raise the family Aspidoceratidae to the rank of a superfamily Aspi- doceratoidea, ranging from the earliest Late Callovian to the Early Berriasian Jacobi Zone. The new superfamily includes two families, Aspidoceratidae (Aspidoceratinae, Euaspidoceratinae, Epipeltoceratinae and Hybonoticeratinae), and Peltoceratidae (Peltoceratinae and Gregoryceratinae nov. subfam.). The highly differentiated features of the aspidoceratoids indicate that their life-histories -

301083243.Pdf
AMEGHINIANA ISSN 0002-7014 Revista de la Asociación Paleontológica Argentina Tomo XII Diciembre de 1975 N?4 THE INDO-PACIFIC AMMONITE MAY AITES IN THE OXFORDIAN OF THESOUTHERN ANDES By P. N. STIPANICIC', G. E. G. WESTERMANN 2 and A. C. RJCCARDI3 ABSTRACT: Oxfordian Iitho- and biostratigraphy of the Chilean and Argentine Andes is reviewed (P. N. Stipanicic). Within the Chacay Group, the Lower to basal Upper Oxfordian La Manga Formation, below, mostly detrital and biogenic, and the Upper Oxfordian Au- quilco Formation, above, mainly chemical, are distinguished. The La Manga Formation (with Gryphaea calceola lumachelle) is rich in ammonite faunas, particularly of thc upper Cordatum to lower Canaliculatum Zones. In Neuquén and Mendoza provinces of Argentina, the Pli- catilis Zone or Middle Oxfordian has yielded Perísphinctes spp., Euaspidoceras spp., Aspido- ceras spp., together with Mayaítes (Araucanites ) stípanícfcí, M. (A.) reyesi, and M. (A.) mulai, Westermann et Riccardi subgen. et spp. nov. The first find of Mayaitidae outside the Indo-Pacific province is discussed in light of _plate-tectonic theory. RESUMEN: La revisron Iito- y bioestratigráfica del Oxfordiano de los Andes de Argentina y Chile (P. N. Stipanicic) ha permitido reconocer dentro del Grupo Chacay: 1) abajo, la Formación La Manga, mayormente detrítica y biogénica, del Oxfordiano inferior-superior basa!, y 2) arriba, la Formación Auquilco, mayormente química, del Oxfordiano superior. La For- mación La Manga (con lumachelas de Gryphaea calceola) contiene abundante cantidad de amonitas, particularmente de las Zonas de Cordatum superior a Canaliculatum inferior. En las provincias de Mendoza y Neuquén,Argentina, la Zona de Plicatilis (Oxfordiano medio) contiene Perispbinctes spp., Euaspidoceras spp., Aspidoceras spp., conjuntamente con Mayaites (Araucanites) stipanicici, M. -

Geology of the Shepton Mallet Area (Somerset)
Geology of the Shepton Mallet area (Somerset) Integrated Geological Surveys (South) Internal Report IR/03/94 BRITISH GEOLOGICAL SURVEY INTERNAL REPORT IR/03/00 Geology of the Shepton Mallet area (Somerset) C R Bristow and D T Donovan Contributor H C Ivimey-Cook (Jurassic biostratigraphy) The National Grid and other Ordnance Survey data are used with the permission of the Controller of Her Majesty’s Stationery Office. Ordnance Survey licence number GD 272191/1999 Key words Somerset, Jurassic. Subject index Bibliographical reference BRISTOW, C R and DONOVAN, D T. 2003. Geology of the Shepton Mallet area (Somerset). British Geological Survey Internal Report, IR/03/00. 52pp. © NERC 2003 Keyworth, Nottingham British Geological Survey 2003 BRITISH GEOLOGICAL SURVEY The full range of Survey publications is available from the BGS Keyworth, Nottingham NG12 5GG Sales Desks at Nottingham and Edinburgh; see contact details 0115-936 3241 Fax 0115-936 3488 below or shop online at www.thebgs.co.uk e-mail: [email protected] The London Information Office maintains a reference collection www.bgs.ac.uk of BGS publications including maps for consultation. Shop online at: www.thebgs.co.uk The Survey publishes an annual catalogue of its maps and other publications; this catalogue is available from any of the BGS Sales Murchison House, West Mains Road, Edinburgh EH9 3LA Desks. 0131-667 1000 Fax 0131-668 2683 The British Geological Survey carries out the geological survey of e-mail: [email protected] Great Britain and Northern Ireland (the latter as an agency service for the government of Northern Ireland), and of the London Information Office at the Natural History Museum surrounding continental shelf, as well as its basic research (Earth Galleries), Exhibition Road, South Kensington, London projects. -

The Barremian Heteromorph Ammonite Dissimilites from Northern Italy: Taxonomy and Evolutionary Implications
The Barremian heteromorph ammonite Dissimilites from northern Italy: Taxonomy and evolutionary implications ALEXANDER LUKENEDER and SUSANNE LUKENEDER Lukeneder, A. and Lukeneder, S. 2014. The Barremian heteromorph ammonite Dissimilites from northern Italy: Taxon- omy and evolutionary implications. Acta Palaeontologica Polonica 59 (3): 663–680. A new acrioceratid ammonite, Dissimilites intermedius sp. nov., from the Barremian (Lower Cretaceous) of the Puez area (Dolomites, northern Italy) is described. Dissimilites intermedius sp. nov. is an intermediate form between D. dissimilis and D. trinodosum. The new species combines the ribbing style of D. dissimilis (bifurcating with intercalating single ribs) with the tuberculation style of D. trinodosum (trituberculation on entire shell). The shallow-helical spire, entirely comprising single ribs intercalated by trituberculated main ribs, is similar to the one of the assumed ancestor Acrioceras, whereas the increasing curvation of the younger forms resembles similar patterns observed in the descendant Toxoc- eratoides. These characters support the hypothesis of a direct evolutionary lineage from Acrioceras via Dissimilites to Toxoceratoides. D. intermedius sp. nov. ranges from the upper Lower Barremian (Moutoniceras moutonianum Zone) to the lower Upper Barremian (Toxancyloceras vandenheckii Zone). The new species allows to better understand the evolu- tion of the genus Dissimilites. The genus appears within the Nicklesia pulchella Zone represented by D. duboise, which most likely evolved into D. dissimilis. In the Kotetishvilia compressissima Zone, two morphological forms developed: smaller forms very similar to Acrioceras and forms with very long shaft and juvenile spire like in D. intermedius sp. nov. The latter most likely gave rise to D. subalternatus and D. trinodosum in the M. -

From the Thesaurus of the Museum Collections. I. Liassic Ammonites from Munteana (Sviniţa Zone, Southern Carpathians, Romania)
ACTA PALAEONTOLOGICA ROMANIAE V. 5 (2005), P. 49-65 FROM THE THESAURUS OF THE MUSEUM COLLECTIONS. I. LIASSIC AMMONITES FROM MUNTEANA (SVINIŢA ZONE, SOUTHERN CARPATHIANS, ROMANIA) John H. CALLOMON1 and Eugen GRĂDINARU2 Abstract: The taxonomy of the Liassic ammonites from Munteana (Sviniţa Zone, South Carpathians, Romania) collected by Răileanu (1953), with some subsequent additions, preserved in the collections of the Department of Geology in the Faculty of Geology and Geophysics, University of Bucharest, is reviewed. The most important elements are represented by the Liparoceratidae. These include forms hitherto unknown, described now as Liparoceras carpathicum sp.nov. [M] and Aegoceras carpathicum sp.nov. [m]. These liparoceratids reveal the presence of a sharply-constrained biohorizon in the Liassic deposits of Munteana of Pliensbachian, Late Carixian age (Davoei Zone) and provide an important palaeobiogeographic reference-point in the region of the Carpathians. The Liassic ammonite fauna from Munteana in Răileanu's collection illustrates the value of the fossils that are locked in museum collections, waiting to be described. Keywords: Răileanu's collection, Ammonites, Liassic, Liparoceratidae, Munteana, South Carpathians, Romania. INTRODUCTION stratigraphic provenance. Conversely, Răileanu's collection from Munteana contain ammonites that The Liassic deposits, which unconformably were never mentioned in his papers. These overlie older formations, have an important ammonites are entirely new, not only to the Banat development in the South Carpathians on the region but also to the Carpathians as a whole. Romanian territory, both in the Getic Nappe and They are thus highly significant for the local and the Danubian Autochthone (Patrulius 1972, regional biostratigraphy and palaeobiogeography Patrulius & al. 1972, Patrulius & Popa 1972). -
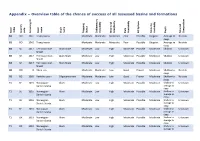
Appendix – Overview Table of the Chance of Success of All Assessed Basins and Formations
Appendix – Overview table of the chance of success of all assessed basins and formations ID ineral ineral Basin index Country code Screening Basin name Shale name Mapping Status Sedimentary Variability Structural Complexity Data Availability System HC Maturity Variability Depth M Composition B1 RO 1041 Transylvania Moderate Moderate Moderate Poor Possible Biogenic Average to No data deep B1 RO 1042 Transylvania Moderate Moderate Moderate Poor Possible Biogenic Average to No data deep B2 SE 1017 Fennoscandian Alum Shale Moderate Low High Moderate Possible Moderate Shallow Unknown Shield B2 SE 1017 Fennoscandian Alum Shale Moderate Low High Moderate Possible Moderate Shallow Unknown Shield B2 SE 1017 Fennoscandian Alum Shale Moderate Low High Moderate Possible Moderate Shallow Unknown Shield O1 RO 0 Black_sea Moderate Moderate Low Good Proven Moderate Shallow to No data deep O1 BG 1060 Kamchia basin Oligocene shale Moderate Moderate Low Good Proven Moderate Shallow to No data deep T1 SE 1015 Norwegian- Alum Moderate Low High Moderate Possible Moderate Shallow to Unknown Danish-Scania average to deep T1 SE 1015 Norwegian- Alum Moderate Low High Moderate Possible Moderate Shallow to Unknown Danish-Scania average to deep T1 SE 1016 Norwegian- Alum Moderate Low High Moderate Possible Moderate Shallow to Unknown Danish-Scania average to deep T1 SE 1016 Norwegian- Alum Moderate Low High Moderate Possible Moderate Shallow to Unknown Danish-Scania average to deep T1 DK 1019 Norwegian- Alum Moderate Low High Moderate Possible Moderate Shallow to -

Geological Magazine
Vol. LXXXVII. No. 2. March-April, 1950 V GEOLOGICAL MAGAZINE Edited by O. M. B. BULMAN and S. R. NOCKOLDS assisted by PROFESSOR W. S. BOULTON PROFESSOR O. T. JONES PROFESSOR W. G. FEARNSIDES PROFESSOR W. J. PUGH PROFESSOR LEONARD HAWKES PROFESSOR C. E. TILLEY HENRY WOODS CONTENTS PAGE OBrruARY—R. H. Rastall, 1871-1950 73 SPATH, L. F. The Study of Ammonites in Thin, Median Sections . 77 CHAPMAN, R. W. Contact Metamorphism at Maddocks Hill near Wellington, Shropshire 85 PERRIN, R., and ROUBAULT, M. Metamorphism of the Trias in the Alps 89 EMERY, K. O. A Suggested Origin of Continental Slopes and of Submarine Canyons 102 JOYCE, J. R. F. Stone Runs of the Falkland Islands . .105 JOHNSON, M. R. W. The Fauna of the Rhaetic Beds in South Nottinghamshire 116 SADASHIVAIAH, M. S. Olivine-bearing and other Basic Hornfelses around the Insch Igneous Mass, Aberdeenshire . .121 GREENLY, E. The Tectonics of Holland Arms 131 WATERSTON, C. D. Note on the Sandstone Injections of Eathie Haven, Cromarty 133 CORRESPONDENCE Investigation of the Torridonian, W. Q. Kennedy and J. E. Hemingway, 139 ; Kent's Cavern, Torquay, E. Pyddoke, 139 ; Pre-Cambrian Formations of India, L. L. Fermor, 140 ; Oceanic Meanderings, G. M. Lees, 144. REVIEWS pages 146-8 STEPHEN AUSTIN & SONS, LTD. 1 FORE STREET, HERTFORD, HERTS SUBSCRIPTION 3°/- PER ANNUM 2/OS.O Q U A G IOC A Ammonites in Thin Section 77 The Study of Ammonites in Thin, Median Sections By L. F. SPATH 1 ABSTRACT During the preparation of the second part of the Catalogue of Ammonoidea of the Trias, several hundred thin median sections of ammonites were prepared and examined.