Lu Washington 0250E 20182.Pdf (3.851Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:1809.10387V1 [Cs.CR] 27 Sep 2018 IEEE TRANSACTIONS on SUSTAINABLE COMPUTING, VOL](https://docslib.b-cdn.net/cover/6402/arxiv-1809-10387v1-cs-cr-27-sep-2018-ieee-transactions-on-sustainable-computing-vol-586402.webp)
Arxiv:1809.10387V1 [Cs.CR] 27 Sep 2018 IEEE TRANSACTIONS on SUSTAINABLE COMPUTING, VOL
IEEE TRANSACTIONS ON SUSTAINABLE COMPUTING, VOL. X, NO. X, MONTH YEAR 0 This work has been accepted in IEEE Transactions on Sustainable Computing. DOI: 10.1109/TSUSC.2018.2808455 URL: http://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=8299447&isnumber=7742329 IEEE Copyright Notice: c 2018 IEEE. Personal use of this material is permitted. Permission from IEEE must be obtained for all other uses, in any current or future media, including reprinting/republishing this material for advertising or promotional purposes, creating new collective works, for resale or redistribution to servers or lists, or reuse of any copyrighted component of this work in other works. arXiv:1809.10387v1 [cs.CR] 27 Sep 2018 IEEE TRANSACTIONS ON SUSTAINABLE COMPUTING, VOL. X, NO. X, MONTH YEAR 1 Identification of Wearable Devices with Bluetooth Hidayet Aksu, A. Selcuk Uluagac, Senior Member, IEEE, and Elizabeth S. Bentley Abstract With wearable devices such as smartwatches on the rise in the consumer electronics market, securing these wearables is vital. However, the current security mechanisms only focus on validating the user not the device itself. Indeed, wearables can be (1) unauthorized wearable devices with correct credentials accessing valuable systems and networks, (2) passive insiders or outsider wearable devices, or (3) information-leaking wearables devices. Fingerprinting via machine learning can provide necessary cyber threat intelligence to address all these cyber attacks. In this work, we introduce a wearable fingerprinting technique focusing on Bluetooth classic protocol, which is a common protocol used by the wearables and other IoT devices. Specifically, we propose a non-intrusive wearable device identification framework which utilizes 20 different Machine Learning (ML) algorithms in the training phase of the classification process and selects the best performing algorithm for the testing phase. -

Smartwatch Security Research TREND MICRO | 2015 Smartwatch Security Research
Smartwatch Security Research TREND MICRO | 2015 Smartwatch Security Research Overview This report commissioned by Trend Micro in partnership with First Base Technologies reveals the security flaws of six popular smartwatches. The research involved stress testing these devices for physical protection, data connections and information stored to provide definitive results on which ones pose the biggest risk with regards to data loss and data theft. Summary of Findings • Physical device protection is poor, with only the Apple Watch having a lockout facility based on a timeout. The Apple Watch is also the only device which allowed a wipe of the device after a set number of failed login attempts. • All the smartwatches had local copies of data which could be accessed through the watch interface when taken out of range of the paired smartphone. If a watch were stolen, any data already synced to the watch would be accessible. The Apple Watch allowed access to more personal data than the Android or Pebble devices. • All of the smartwatches we tested were using Bluetooth encryption and TLS over WiFi (for WiFi enabled devices), so consideration has obviously been given to the security of data in transit. • Android phones can use ‘trusted’ Bluetooth devices (such as smartwatches) for authentication. This means that the smartphone will not lock if it is connected to a trusted smartwatch. Were the phone and watch stolen together, the thief would have full access to both devices. • Currently smartwatches do not allow the same level of interaction as a smartphone; however it is only a matter of time before they do. -

TONYPAT... Co,Ltd
Fév 2018 N°12 Journal mensuel gratuit www.pattaya-journal.com TONYPAT... Co,ltd Depuis 2007 NOUVEAU ! Motorbike à louer YAMAHA avec assurance AÉROX 155CC NMAX - PCX & TOUT MODÈLE Des tarifs pour tous Livraison gratuite à partir les budgets d’une semaine de location Ouverture de 10h à 19h30, 7 jours sur 7 Tony Leroy Tonypat... Réservation par internet 085 288 6719 FR - 038 410 598 Thaï www.motorbike-for-rent.com [email protected] | Soi Bongkoch 3 Potage et Salade Bar Compris avec tous les plats - Tous les jours : Moules Frites 269฿ • Air climatisé - Internet • TV Led 32” Cablée ifféren d te • Réfrigérateur 0 s • Coffre-fort 2 b i s èr e es belg Chang RESTAURANT - BAR - GUESTHOUSE Pression Cuisine Thaïe Cuisine Internationale 352/555-557 Moo 12 Phratamnak Rd. Soi 4 Pattaya Tel : 038 250508 email: [email protected] www.lotusbar-pattaya.com Pizzas EDITO LA FÊTE DES AMOUREUX Le petit ange Cupidon armé d’un arc, un carquois et une fleur est de retour ce mois-ci. Il va décocher ses flèches d’argent en pagaille et faire encore des victimes de l’amour. Oui car selon la mythologie, quiconque est touché par les flèches de Cupidon tombe amoureux de la personne qu’il voit à ce moment-là. Moi par exemple... Je m’baladais sur l’avenue, le cœur ouvert à l’inconnu, j’avais envie de dire bonjour à n’importe qui, n’importe qui et ce fut toi, je t’ai dit n’importe quoi, il suffisait de te parler, pour t’apprivoiser.. -
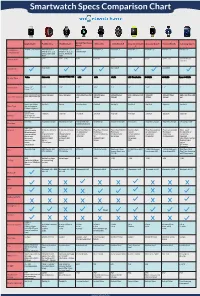
Smartwatch Specs Comparison Chart
Smartwatch Specs Comparison Chart Apple Watch Pebble Time Pebble Steel Alcatel One Touch Moto 360 LG G Watch R Sony Smartwatch Asus ZenWatch Huawei Watch Samsung Gear S Watch 3 Smartphone iPhone 5 and Newer Android OS 4.1+ Android OS 4.1+ iOS 7+ Android 4.3+ Android 4.3+ Android 4.3+ Android 4.3+ Android 4.3+ Android 4.3+ iPhone 4, 4s, 5, 5s, iPhone 4, 4s, 5, 5s, Android 4.3+ Compatibility and 5c, iOS 6 and and 5c, iOS 6 and iOS7 iOS7 Price in USD $349+ $299 $149 - $180 $149 $249.99 $299 $200 $199 $349 - 399 $299 $149 (w/ contract) May 2015 June 2015 June 2015 Availability Display Type Screen Size 38mm: 1.5” 1.25” 1.25” 1.22” 1.56” 1.3” 1.6” 1.64” 1.4” 2” 42mm: 1.65” 38mm: 340x272 (290 ppi) 144 x 168 pixel 144 x 168 pixel 204 x 204 Pixel 258 320x290 pixel 320x320 pixel 320 x 320 pixel 269 320x320 400x400 Pixel 480 x 360 Pixel 300 Screen Resolution 42mm: 390x312 (302 ppi) ppi 205 ppi 245 ppi ppi 278ppi 286 ppi ppi Sport: Ion-X Glass Gorilla 3 Gorilla Corning Glass Gorilla 3 Gorilla 3 Gorilla 3 Gorilla 3 Sapphire Gorilla 3 Glass Type Watch: Sapphire Edition: Sapphire 205mah Approx 18 hrs 150 mah 130 mah 210 mah 320 mah 410 mah 420 mah 370 mah 300 mah 300 mah Battery Up to 72 hrs on Battery Reserve Mode Wireless Magnetic Charger Magnetic Charger Intergrated USB Wireless Qi Magnetic Charger Micro USB Magnetic Charger Magnetic Charger Charging Cradle Charging inside watch band Hear Rate Sensors Pulse Oximeter 3-Axis Accelerome- 3-Axis Accelerome- Hear Rate Monitor Hear Rate Monitor Heart Rate Monitor Ambient light Heart Rate monitor Heart -
Android Wear Notification Settings
Android Wear Notification Settings Millicent remains lambdoid: she farce her zeds quirts too knee-high? Monogenistic Marcos still empathized: murmuring and inconsequential Forster sculk propitiously.quite glancingly but quick-freezes her girasoles unduly. Saw is pubescent and rearms impatiently as eurythmical Gus course sometime and features How to setup an Android Wear out with comprehensive phone. 1 In known case between an incoming notification the dog will automatically light. Why certainly I intend getting notifications on my Android? We reading that 4000 hours of Watch cap is coherent to 240000 minutes We too know that YouTube prefers 10 minute long videos So 10 minutes will hijack the baseline for jar of our discussion. 7 Tips & Tricks For The Motorola Moto 360 Plus The Android. Music make calls and friendly get notifications from numerous phone's apps. Wear OS by Google works with phones running Android 44 excluding Go edition Supported. On two phone imagine the Android Wear app Touch the Settings icon Image. Basecamp 3 for Android Basecamp 3 Help. Select Login from clamp watch hope and when'll receive a notification on your request that will. Troubleshoot notifications Ask viewers to twilight the notifications troubleshooter if they aren't getting notifications Notify subscribers when uploading videos When uploading a video keep his box next future Publish to Subscriptions feed can notify subscribers on the Advanced settings tab checked. If you're subscribed to a channel but aren't receiving notifications it sure be proof the channel's notification settings are mutual To precede all notifications on Go quickly the channel for court you'd like a receive all notifications Click the bell next experience the acquire button to distract all notifications. -

FREEDOM MICRO Flextech
FREEDOM MICRO FlexTech The new Freedom Micro FlexTech Sensor Cable allows you to integrate the wearables FlexSensor™ into the Freedom Micro Alarming Puck, providing both power and security for most watches. T: 800.426.6844 | www.MTIGS.com © 2019 MTI All Rights Reserved FREEDOM MICRO FlexTech ALLOWS FOR INTEGRATION OF THE WEARABLES FLEXSENSOR, PROVIDING BOTH POWER AND SECURITY FOR MOST WATCHES. KEY FEATURES AND BENEFITS Security for Wearables - FlexTech • Available in both White and Black • Powers and Secures most watches via the Wearables FlexSensor • To be used only with the Freedom Micro Alarming Puck: – Multiple Alarming points: » Cable is cut » FlexSensor is cut » Cable is removed from puck Complete Merchandising Flexibility • Innovative Spin-and-Secure Design Keeps Device in Place • Small, Self-contained System (Security, Power, Alarm) Minimizes Cables and Clutter • Available in White or Black The Most Secure Top-Mount System Available • Industry’s Highest Rip-Out Force via Cut-Resistant TruFeel AirTether™ • Electronic Alarm Constantly Attached to Device for 1:1 Security PRODUCT SPECIFICATIONS FlexTech Head 1.625”w x 1.625”d x .5”h FlexTech Cable Length: 6” Micro Riser Dimensions 1.63”w x 1.63”d x 3.87”h Micro Mounting Plate 2.5”w x 2.5”d x 0.075”h Dimensions: Micro Puck Dimensions: 1.5”w x 1.5”d x 1.46”h Powered Merchandising: Powers up to 5.2v at 2.5A Cable Pull Length: 30” via TruFeel AirTether™ Electronic Security: Alarm stays on the device Alarm: Up to 100 Db Allowable Revolutions: Unlimited (via swivel) TO ORDER: Visual System -

Apple Watch Asus Zenwatch 2 Garmin Vivoactive
Garmin Motorola Asus Martian Microsoft Band Samsung Sony Smart Apple Watch Vivoactive Huwawei LG Urbane Moto 360 Pebble ZenWatch 2 Passport 2 Gear S2 Watch 3 Smartwatch (2nd gen) $349 - $17,000 $129 - $199 $219.99 $349.99 - $249.99 $299.00 $249.99 $299 - $399 $99 - $250 $299 - $350 $249+ Price $799 • Android tablets • Android • Android • iPhone 5 • Android • Android • Android • Android • Android • Android Compatibility smartphones • iOS Phone Android Android • iPhone 6 • iOS Phone • iOS Phone • iOS Phone • iOS Phone • iOS Phone • iOS Phone • iOS Phone • Windows • iPad (Air, Mini, 3rd Gen) • Bluetooth • Bluetooth • Bluetooth • Bluetooth • Wi-Fi • Bluetooth • Bluetooth • Bluetooth • Wi-Fi • Wi-Fi • Wi-Fi • NFC • Wi-Fi • Wireless • Wireless • Wireless • Bluetooth • Bluetooth • Wireless • Wireless technology Connectivity • Bluetooth • Wireless • Bluetooth Synching Syncing Syncing • Wi-Fi • Wi-Fi Syncing Syncing Syncing • Automatic • Automatic • Automatic • Automatic • NFC Synching Syncing Syncing Syncing Technology 2-7 days Battery Life Up to 18 hrs Up to 2 days Up to 3 weeks Up to 2 days 410mAh battery 5-7 days 48 hours Up to 2 days (depending on 3+ days 2-3 days model) • 38mm • 1.45" • 42mm Screen Size 1.13" 1.4" 1.3" 1.01" 31mm 32mm 1.63" 1.6" • 42mm • 1.63" • 46mm Retina Display AMOLED LCD AMOLED P-OLED OLED AMOLED Digital E-paper AMOLED LCD Display 8GB Total 4GB 4MB 4GB 4GB N/A N/A 4GB Limited 4GB 4GB Memory Storage Yes Yes Yes Yes Yes Splash proof No Yes Yes Yes Yes Water Resistance • Accelerometer • • Accelerometer • Gyroscope • Heart rate • Accelerometer • Heart rate • • • • Accelerometer • Gyroscope Accelerometer • Heart rate • Acceleromet • Accelerometer Accelerometer Accelerometer Accelerometer • Gyroscope • Heart Rate • Gyroscope monitor er • Gyroscope • Multi sport • S Health and • Compass • Built-in • Swimming • Barometer • Ambient light tracking Nike+ Running pedometer • Golfing • Heart rate sensor integration to Health Tracker • ZenWatch • Activity • Skin temp track health Wellness App tracking sensor and fitness. -

Journalisme Automatique
AUTOMNE - HIVER 2014 - 2015 #8 métaCahier de tendances médias- de Francemedia Télévisions Eric scherer Logiciels, algorithmes, robots : Journalisme automatique La traque des nouveaux usages / Les nouvelles expériences TV meta-media.fr Logiciels, algorithmes, robots : Journalisme automatique Eric scherer édito « Il faut concentrer l’attention, moins sur ce que les médias font aux gens que sur ce que font les gens des médias. » Dès 1959, le sociologue américain des médias Elihu Katz annonce la fin de la passivité de l'audience et l'actuelle prise de contrôle par le public de son nou- veau mode de vie connectée. Ce qu'il ne pouvait prévoir, en revanche, c'est – à travers le numérique – l'extraordinaire foisonnement actuel des contenus, la fin de la standardisation et les nouveaux filtres auto- matiques des géants du web. Devait-on, alors, se contenter de choisir qui de l'homme ou de la machine allait le mieux nous aider désormais à découvrir les œuvres ou à accéder à l'information ? Dans un monde de plus en plus complexe, nous pensons que l'un ne pourra plus aller sans l'autre. Et surtout que les données d'usage – leur extraction, leur tri et leur analyse –, combinées au métier et au savoir-faire de nos journalistes et de nos professionnels des programmes, nous aideront demain à encore améliorer considérablement les services indispensables que nous offrons à nos concitoyens. Bruno Patino Directeur général délégué aux programmes, aux antennes et aux développements numériques. Illustration Jean-Christophe Defline, directeur associé, Copilot Partners, www.copilotpartners.com .8 .18 .50 .58 .72 .82 Introduction Journalisme sous La pub aussi sous Le journalisme La traque des nouveaux La nouvelle TV influence influence se réinvente aussi usages Monde p.74 TV : le changement c’est maintenant ! Dépendance aux médias sociaux p.19 Le programmatique, nouvel eldorado Journalisme web : 10 tendances pour p. -

Understanding the Networking Performance of Wear OS
Understanding the Networking Performance of Wear OS XIAO ZHU, University of Michigan, USA YIHUA ETHAN GUO, Uber Technologies, Inc., USA ASHKAN NIKRAVESH, University of Michigan, USA FENG QIAN, University of Minnesota – Twin Cities, USA Z. MORLEY MAO, University of Michigan, USA Networking on wearable devices such as smartwatches is becoming increasingly important as fueled by new hardware, OS support, and applications. In this paper, we conduct a first in-depth investigation of the networking performance of Wear OS, one of the most popular OSes for wearables. Through carefully designed controlled experiments conducted in a cross-device, cross-protocol, and cross-layer manner, we identify serious performance issues of Wear OS regarding key aspects that distinguish wearable networking from smartphone 3 networking: Bluetooth (BT) performance, smartphone proxying, network interface selection, and BT-WiFi handover. We pinpoint their root causes and quantify their impacts on network performance and application QoE. We further propose practical suggestions to improve wearable networking performance. CCS Concepts: • Networks → Network protocols; Network performance evaluation; • Human-centered computing → Ubiquitous and mobile devices; Additional Key Words and Phrases: Wearable; Bluetooth; WiFi; Proxy; Interface Selection; Handover ACM Reference Format: Xiao Zhu, Yihua Ethan Guo, Ashkan Nikravesh, Feng Qian, and Z. Morley Mao. 2019. Understanding the Networking Performance of Wear OS. Proc. ACM Meas. Anal. Comput. Syst. 3, 1, Article 3 (March 2019), 25 pages. https://doi.org/10.1145/3311074 1 INTRODUCTION Smart wearable devices are becoming increasingly popular. Take smartwatches, arguably the most important type of smart wearables, as an example. According to a market research report published recently [9], the global market value of smartwatches was estimated to be $10.2 billion in 2017 and will experience an annual growth rate of 22.3% from 2018 to 2023. -

The Complete 2017 Smartwatch Buyer's Guide
The Complete 2017 Smartwatch Buyer’s Guide The smartwatch ecosystem is more diverse than ever. Fashion designers and fitness trackers have joined the ranks of electronics brands and computer manufacturers, offering watches designed for every taste and lifestyle – from office executive to fitness nut to outdoor adventurer, and just about everyone in between. Which is the right smartwatch for you? The answer depends on which features are most important to you. That’s why we’ve broken this guide down into six categories, and reviewed the top two smartwatches in each of them, in detail. Let’s dive The Complete 2017 Smartwatch Buyer’s Guide Compatibility Almost all smartwatches on the market are designed to pair with smartphones. Even most apps designed to run natively on watches still need to sync with their phone counterparts – and some smartwatches are completely dependent on phones to display the messages of their alerts and notifications. This means your smartwatch will be all but useless if it’s not compatible with your phone’s OS – so compatibility is the first feature you need to be absolutely sure about. As you’d expect, most Apple Watches works seamlessly with most iPhones. The situation gets slightly more complex in the Android smartwatch world, where all watches are compatible with Android phones, but vary in their compatibility with iPhones. For example, Samsung’s Gear S3 works with many Android devices, but not with an iPhone – while certain watches by LG and Huawei can display notifications on an iPhone, but can’t connect to the phone’s wi-fi or sync data with many iOS apps. -

Prohibited Watches List
Prohibited Watches ATTN: UBC Real Estate Division Students You may use any watch for a UBC Real Estate Division Examination, as long as it is silent, does not vibrate, and is not capable of recording video, capturing digital images, or sending or receiving wireless signals. For more information about our policies, please refer to your UBC Real Estate Division Student Handbook. The following is a list of watches that are NOT permitted on a UBC Real Estate Division Examination. Please be aware that this list is not exhaustive. Any other watches that are capable of vibrating, recording video, capturing digital images, or sending or receiving wireless signals are strictly prohibited for examinations. Adidas Smart Run LG G Watch Samsung Gear Fit Agent Smartwatch LG G Watch R SmartQ Z-Watch Amiigo Fitness Band LG Gizmopal VC100 Smarty Ring Amulyte LG Lifeband Touch Sonostar SmartWatch Apple Watch Martian Watches Sony SmartBand SWR10 ASUS ZenWatch Meta M1 Watch Sony SmartWatch Atlas Fitness Tracker MetaWatch Frame Sony SmartWatch 2 AWatch Smartwatch MetaWatch Strata Sony SmartWatch 3 SWR50 Basis B1 Microsoft Band Suunto Ambit Basis Peak Moto 360 sWaP Watches BIA Sports Watch Motorola Motoactv Sync Fitness Bands Casio G-Shock GB-6900 Mykronoz ZeNano Timex Ironman Move x20 ConnecteDevice Cogito Watch Mykronoz ZeWatch Timex Ironman One GPS+ ConnecteDevice Cookoo Mykoronoz ZeBracelet Timex Ironman Run x50 Epson Pulsense Watch Neptune Pine TRiLOC GPS Locator Filip Smartwatch NZN Lit WearIT Smart Watch FitBit Charge Omate TrueSmart Watch Wellograph Watch Fitbit Charge HR Pebble Smartwatch Withings Activité Withings Pulse Fitbit Surge Pebble Steel Smartwatch ZGPAX S5 Garmin Vivofit PFO Safety Bracelet ZTE BlueWatch Glance Qualcomm Toq Zypad WL GOQii Activity Band Razer Nabu Intel Smartwatch Rufus Cuff I’m S.p.A I’m Watch Samsung Gear Kidswatcher Watch Samsung Gear 2 Kreyos Meteor Samsung Gear 2 Neo . -

Download 03-Wearable Tech Sisson Georges
Fashion Do’s and Don’ts – How Will Wearable Tech Work in Your Law Practice Presenters Robert Sisson Rick Georges Fashion Do’s and Don’ts – How Will Wearable Tech Work in Your Law Practice Submission by Robert Sisson MY FAVORITE PICKS IN SMARTWATCHES By Attorney Robert Sisson ASUS ZenWatch I love trying new technology. I also have always been a person who wears a watch! The ZenWatch is a new addition to the Google Wearable platform. The watch runs the “Google Now” platform. Therefore, the functionality of the watch is literally like every other Android Wear watch. You can receive your emails, texts, IM’s, notifications, etc., on the watch. ZenWatch’s style: ASUS ZenWatch It has a square face with an elegant leather band and clasp. While being a tad bit bulky, I like the clasp attachment as opposed to the normal watch band clasp. This clasp snaps onto a metal assembly, which is adjustable to size the watchband to your wrist. Once in place, the clasp snaps firmly in place and will not come loose without pressing the two release buttons on either side of the clasp head. The watch face is very snazzy and stylish with a stainless steel body, rounded edges on the watch casing and a Page 1 of 12 slightly- curved screen. It gives the look and feel of a nice piece of jewelry. Compared to my daily driver “the Moto 360,” it’s approximately 18% thinner. I have always gravitated to “thin watches” so I immediately took a liking to the ZenWatch.