Hematite Vs. Magnetite As the Signature for Planetary Magnetic Anomalies? Guntherè Kletetschka ), Peter J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Study of Earth's Magnetism
THE STUDY OF EARTH’S MAGNETISM (1269-1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM Vincent Courtillot, Jean-Louis Le Mouël To cite this version: Vincent Courtillot, Jean-Louis Le Mouël. THE STUDY OF EARTH’S MAGNETISM (1269-1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM. Reviews of Geophysics, American Geophysical Union, 2007, 45 (3), pp.RG3008. 10.1029/2006RG000198. insu-02448801 HAL Id: insu-02448801 https://hal-insu.archives-ouvertes.fr/insu-02448801 Submitted on 22 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THE STUDY OF EARTH’S MAGNETISM (1269–1950): A FOUNDATION BY PEREGRINUS AND SUBSEQUENT DEVELOPMENT OF GEOMAGNETISM AND PALEOMAGNETISM Vincent Courtillot1 and Jean-Louis Le Moue¨l1 Received 17 February 2006; revised 14 July 2006; accepted 18 September 2006; published 25 September 2007. [1] This paper summarizes the histories of geomagnetism induced magnetizations), Delesse (remagnetization in a and paleomagnetism (1269–1950). The role of Peregrinus direction opposite to the original), and Melloni (direction of is emphasized. In the sixteenth century a debate on local lava magnetization acquired at time of cooling). -

Download PDF About Minerals Sorted by Mineral Name
MINERALS SORTED BY NAME Here is an alphabetical list of minerals discussed on this site. More information on and photographs of these minerals in Kentucky is available in the book “Rocks and Minerals of Kentucky” (Anderson, 1994). APATITE Crystal system: hexagonal. Fracture: conchoidal. Color: red, brown, white. Hardness: 5.0. Luster: opaque or semitransparent. Specific gravity: 3.1. Apatite, also called cellophane, occurs in peridotites in eastern and western Kentucky. A microcrystalline variety of collophane found in northern Woodford County is dark reddish brown, porous, and occurs in phosphatic beds, lenses, and nodules in the Tanglewood Member of the Lexington Limestone. Some fossils in the Tanglewood Member are coated with phosphate. Beds are generally very thin, but occasionally several feet thick. The Woodford County phosphate beds were mined during the early 1900s near Wallace, Ky. BARITE Crystal system: orthorhombic. Cleavage: often in groups of platy or tabular crystals. Color: usually white, but may be light shades of blue, brown, yellow, or red. Hardness: 3.0 to 3.5. Streak: white. Luster: vitreous to pearly. Specific gravity: 4.5. Tenacity: brittle. Uses: in heavy muds in oil-well drilling, to increase brilliance in the glass-making industry, as filler for paper, cosmetics, textiles, linoleum, rubber goods, paints. Barite generally occurs in a white massive variety (often appearing earthy when weathered), although some clear to bluish, bladed barite crystals have been observed in several vein deposits in central Kentucky, and commonly occurs as a solid solution series with celestite where barium and strontium can substitute for each other. Various nodular zones have been observed in Silurian–Devonian rocks in east-central Kentucky. -

Relationships Between Magnetic Parameters, Chemical Composition and Clay Minerals of Topsoils Near Coimbra, Central Portugal
Nat. Hazards Earth Syst. Sci., 12, 2545–2555, 2012 www.nat-hazards-earth-syst-sci.net/12/2545/2012/ Natural Hazards doi:10.5194/nhess-12-2545-2012 and Earth © Author(s) 2012. CC Attribution 3.0 License. System Sciences Relationships between magnetic parameters, chemical composition and clay minerals of topsoils near Coimbra, central Portugal A. M. Lourenc¸o1, F. Rocha2, and C. R. Gomes1 1Centre for Geophysics, Earth Sciences Dept., University of Coimbra, Largo Marquesˆ de Pombal, 3000-272 Coimbra, Portugal 2Geobiotec Centre, Geosciences Dept., University of Aveiro, 3810-193 Aveiro, Portugal Correspondence to: A. M. Lourenc¸o ([email protected]) Received: 6 September 2011 – Revised: 27 February 2012 – Accepted: 28 February 2012 – Published: 14 August 2012 Abstract. Magnetic measurements, mineralogical and geo- al., 1980). This methodology is fast, economic and can be ap- chemical studies were carried out on surface soil samples plied in various research fields, such as environmental mon- in order to find possible relationships and to obtain envi- itoring, pedology, paleoclimatology, limnology, archeology ronmental implications. The samples were taken over a and stratigraphy. Recent studies have demonstrated the ad- square grid (500 × 500 m) near the city of Coimbra, in cen- vantages and the potential of the environmental magnetism tral Portugal. Mass specific magnetic susceptibility ranges methods as valuable aids in the detection and delimitation between 12.50 and 710.11 × 10−8 m3 kg−1 and isothermal of areas affected by pollution (e.g. Bityukova et al., 1999; magnetic remanence at 1 tesla values range between 253 Boyko et al., 2004; Blaha et al., 2008; Lu et al., 2009; and 18 174 × 10−3 Am−1. -

Lts Ficez 5Coz7'a1ra
ltS FicEz 5coZ7'a1ra he most valuable gem- Gem-quality red beryl on quality red beryl comes white devitri- from thc Wah Wah fied rhyolite Mountains of south- matrix. This western Utah (Fig. 1). sample mea- Red beryl occurs as a secondary sures 9 x 26 mm (O.35 x 1 mineral in topaz rhyolite. Market- in.l. (Photo by able crystals from the Wah Wah Sky Hall.) Mountains have been produced from the Violet Mine, operated on a limited scale since 1976 under the ownership of the Harris family of Delta, UT. From 1989 to 1995. oroduction from the mine amounted to more than $3 million with an inventory of several million dollars of unsold gems (Harris, 1995). In 7994, Kennecott Exploration leased the mine and surrounding claims to de- termine reserves and feasibility of gem recovery. Mining and explora- E.H. Christiansen and tion activity are continuing. J.D. Keith are proles- Previous work on red beryl has s0rs arld T.J. dealt mainly with crystallographic, Thompson is a student, chemical and optical properties of the crystals (Shigley and Foord, Ieparlment of Geohgy, 1984) with only general descriptions Brigham Yrung Univer- of the geology (Ream, 1979). How- sitt Brx 251 I l, Prlvn, ever. the conditions needed for for- mation of red beryl have not been UT 84fi[2 well constrained. It is generally pro- posed that beryl originates by pre- cipitation in fractures and vugs from high-temperature silica rhyolite and andesitic lava flows. gases as they are released from slowly cooled rhyolite Beginning about 23 Ma, the style and composition of lava. -

Compilation of Reported Sapphire Occurrences in Montana
Report of Investigation 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg 2015 Cover photo by Richard Berg. Sapphires (very pale green and colorless) concentrated by panning. The small red grains are garnets, commonly found with sapphires in western Montana, and the black sand is mainly magnetite. Compilation of Reported Sapphire Occurrences, RI 23 Compilation of Reported Sapphire Occurrences in Montana Richard B. Berg Montana Bureau of Mines and Geology MBMG Report of Investigation 23 2015 i Compilation of Reported Sapphire Occurrences, RI 23 TABLE OF CONTENTS Introduction ............................................................................................................................1 Descriptions of Occurrences ..................................................................................................7 Selected Bibliography of Articles on Montana Sapphires ................................................... 75 General Montana ............................................................................................................75 Yogo ................................................................................................................................ 75 Southwestern Montana Alluvial Deposits........................................................................ 76 Specifi cally Rock Creek sapphire district ........................................................................ 76 Specifi cally Dry Cottonwood Creek deposit and the Butte area .................................... -
Formation of Jarosite Minerals in the Presence of Seed Material H
46th Lunar and Planetary Science Conference (2015) 1825.pdf FORMATION OF JAROSITE MINERALS IN THE PRESENCE OF SEED MATERIAL H. E. A. Brand1,2 N. V. Y. Scarlett2 and I. E. Grey2, 1Australian Synchrotron, 800 Blackburn Rd., Clayton South, VIC 3168, Australia. [email protected] 2CSIRO Mineral Resources, Box 312, Clayton, VIC 3187, Australia. Introduction: There has been a resurgence in interest Experimental method: Jarosite forms readily by co- in jarosite, (K,Na)Fe3(SO4)2(OH)6, and related precitation on gentle warming of an appropriate minerals since their detection on Mars by the MER solution. 2.25 g of Fe2(SO4)3.xH2O and 0.70 g of rover Opportunity [1]. In this context, the presence of K2SO4 were dissolved in 10 mls of deionized H2O. jarosite has been recognised as a likely indicator of Seed materials were diamond, hematite and goethite. water at the surface of Mars in the past and it is hoped Hematite and goethite are commonly found in that study of their formation mechanisms will provide association with natural jarosite deposits. The inclusion insight into the environmental history of Mars [2]. of diamond provides a baseline for the effect of an inert Jarosites are also of great importance to a range of seed material. These materials were added to the mineral processing and research applications. For jarosite starting-solutions to give 5 wt% seed. An example: they are used in the removal of iron species additional solution was prepared with no seed material from smelting processes; they form detrimentally in to provide a comparison set of data. -

The Efficient Improvement of Original Magnetite in Iron Ore Reduction
minerals Article The Efficient Improvement of Original Magnetite in Iron Ore Reduction Reaction in Magnetization Roasting Process and Mechanism Analysis by In Situ and Continuous Image Capture Bing Zhao 1,2, Peng Gao 1,2,*, Zhidong Tang 1,2 and Wuzhi Zhang 1,2 1 School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China; [email protected] (B.Z.); [email protected] (Z.T.); [email protected] (W.Z.) 2 National-Local Joint Engineering Research Center of High-Efficient Exploitation Technology for Refractory Iron Ore Resources, Shenyang 110819, China * Correspondence: [email protected]; Tel.: +86-024-8368-8920 Abstract: Magnetization roasting followed by magnetic separation is considered an effective method for recovering iron minerals. As hematite and magnetite are the main concomitant constituents in iron ores, the separation index after the magnetization roasting will be more optimized than with only hematite. In this research, the mechanism of the original magnetite improving iron ore reduction during the magnetization roasting process was explored using ore fines and lump ore samples. Under optimum roasting conditions, the iron grade increased from 62.17% to 65.22%, and iron recovery increased from 84.02% to 92.02% after separation, when Fe in the original magnetite content increased from 0.31% to 8.09%, although the Fe masses in each sample were equal. For lump ores with magnetite and hematite intergrowth, the method of in situ and continuous image capture Citation: Zhao, B.; Gao, P.; Tang, Z.; for microcrack generation and the evolution of the magnetization roasting process was innovatively Zhang, W. -

Recovery of Magnetite-Hematite Concentrate from Iron Ore Tailings
E3S Web of Conferences 247, 01042 (2021) https://doi.org/10.1051/e3sconf/202124701042 ICEPP-2021 Recovery of magnetite-hematite concentrate from iron ore tailings Mikhail Khokhulya1,*, Alexander Fomin1, and Svetlana Alekseeva1 1Mining Institute of Kola Science Center of Russian Academy of Sciences, Apatity, 184209, Russia Abstract. The research is aimed at study of the probable recovery of iron from the tailings of the Olcon mining company located in the north-western Arctic zone of Russia. Material composition of a sample from a tailings dump was analysed. The authors have developed a separation production technology to recover magnetite-hematite concentrate from the tailings. A processing flowsheet includes magnetic separation, milling and gravity concentration methods. The separation technology provides for production of iron ore concentrate with total iron content of 65.9% and recovers 91.0% of magnetite and 80.5% of hematite from the tailings containing 20.4% of total iron. The proposed technology will increase production of the concentrate at a dressing plant and reduce environmental impact. 1 Introduction The mineral processing plant of the Olcon JSC, located at the Murmansk region, produces magnetite- At present, there is an important problem worldwide in hematite concentrate. The processing technology the disposal of waste generated during the mineral includes several magnetic separation stages to produce production and processing. Tailings dumps occupy huge magnetite concentrate and two jigging stages to produce areas and pollute the environment. However, waste hematite concentrate from a non-magnetic fraction of material contains some valuable components that can be magnetic separation [13]. used in various industries. In the initial period of plant operation (since 1955) In Russia, mining-induced waste occupies more than iron ore tailings were stored in the Southern Bay of 300 thousand hectares of lands. -

Banded Iron Formations
Banded Iron Formations Cover Slide 1 What are Banded Iron Formations (BIFs)? • Large sedimentary structures Kalmina gorge banded iron (Gypsy Denise 2013, Creative Commons) BIFs were deposited in shallow marine troughs or basins. Deposits are tens of km long, several km wide and 150 – 600 m thick. Photo is of Kalmina gorge in the Pilbara (Karijini National Park, Hamersley Ranges) 2 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock Iron rich bands: hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). Iron poor bands: chert (fine‐grained quartz) and low iron oxide levels Rock sample from a BIF (Woudloper 2009, Creative Commons 1.0) Iron rich bands are composed of hematitie (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). The iron poor bands contain chert (fine‐grained quartz) with lesser amounts of iron oxide. 3 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock • Archaean and Proterozoic in age BIF formation through time (KG Budge 2020, public domain) BIFs were deposited for 2 billion years during the Archaean and Proterozoic. There was another short time of deposition during a Snowball Earth event. 4 Why are BIFs important? • Iron ore exports are Australia’s top earner, worth $61 billion in 2017‐2018 • Iron ore comes from enriched BIF deposits Rio Tinto iron ore shiploader in the Pilbara (C Hargrave, CSIRO Science Image) Australia is consistently the leading iron ore exporter in the world. We have large deposits where the iron‐poor chert bands have been leached away, leaving 40%‐60% iron. -
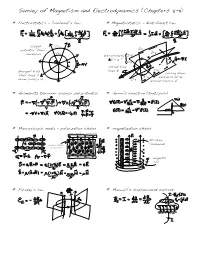
Survey of Magnetism and Electrodynamics (Chapters 5-11)
Survey of Magnetism and Electrodynamics (Chapters 5-11) * Electrostatics - Coulomb’s law * Magnetostatics - Biot-Savart law closed V=1 potential ”flow “ I=1 V=2 I=2 surfaces discontinuity I=0 I = 6 ! I=6 closed flux divergent field lines B I=3 curling flow ”flux “lines D I=4 surfaces of H from source Q I=5 around source I * Helmholtz theorem: source and potential * Green ‘s function (tent/pole) * Macroscopic media - polarization chains * magnetization chains free charge in conductor M-chain bound charge (solenoid) in dielectric magnetic coil * Faraday’s law * Maxwell’s displacement current * Three electrical devices, each the ratio of flux / flow CAPACITOR RESISTOR INDUCTOR * Electrodynamics equations Lorentz force Continuity Maxwell electric, magnetic fields Constitution Potentials (wave equation) Gauge transform * Conserved currents desity flux energy momentum * Electromagnetic waves - Fresnell’s coefficients - skin depth - dipole radiation Section 5.1.1 – Magnetic Fields * the magnetic force was known in antiquity, but was more difficult to quantify ~ predominant effect in nature involves magnetization, not electric currents ~ no magnetic (point) charge (monopole); 1-d currents instead of 0-d charges ~ static electricity was produced in the lab long before steady currents * History: http://maxwell.byu.edu/~spencerr/phys442/node4.html 600 BC Thales of Miletus discovers lodestone’s atraction to iron 1200 AD Chinese use lodestone compass for navigation 1259 AD Petrus Peregrinus (Italy) discovers the same thing 1600 AD William Gilbert -

Magnetic Rocks © Learning A–Z Learning A–Z J Written by Katherine Follett Lexile 470L
FOCUS Book Magnetic Make a magnet! Get a strong magnet and a thin metal bolt. Rub the bolt Rocks across the magnet in one direction fifty times. Pick up a staple with the bolt. You made a magnet! Design a test for your magnet. What can it pick up? What else can you test? Now make another magnet with a new bolt. Change one thing about how you make the magnet. Then repeat your test. How did the results change? Beyond the Book Go to two places with sand. Find out which kind of sand has the most magnetite. Metal and Magnets Magnetic Have you ever played with magnets? Maybe there are magnets on your Rocks refrigerator. Some toys also have magnets in them. A magnet is a special piece of metal. It pulls on, or attracts, other pieces FOCUS Question of metal. What kinds of natural materials stick to magnets? But where do magnets come from? Cause and Effect Photo Credits: Front cover: © Joel Arem/Science Source/Getty Images; page 2: © Vanessa Davies/DK Images; page 3: © Art Directors & TRIP/Alamy Stock Photo; page 4: © Richard Hutchings/Science Source; page 5 (top): © Charles O’Rear/Corbis/VCG/Corbis Documentary/Getty Images; page 5 (bottom): © David Lee/ Alamy Stock Photo; page 6 (left): Sarah Cebulski/© Learning A–Z; page 6 (right): © Alexandru Dobrea/ Hemera/Thinkstock; page 7 (top): © Visuals Unlimited/Getty Images; page 7 (bottom): © Liu Liqun/Corbis Documentary/Getty Images; page 9: © The Natural History Museum/Alamy Stock Photo Illustration Credits: Page 8: Signe Nordin/© Learning A–Z; border (rock): Nora Voutas/© Learning A–Z; (clip): Casey Jones/ © Learning A–Z Reading Levels Magnetic Rocks © Learning A–Z Learning A–Z J Written by Katherine Follett Lexile 470L Correlations All rights reserved. -

Magnetic Properties of Rocks and Minerals
MagneticProperties of Rocksand Minerals ChristopherP. Hunt, BruceM. Moskowitz,Subir K. Banerjee 1. INTRODUCTION composition-dependentmagnetic parameters of a varietyof iron-bearingminerals. This is an updatedcollation of magneticparameters of rocksand mineralsfor geologists,geochemists, and geo- 2. MAGNETIC SUSCEPTIBILITY physicists.Since the publication of theprevious edition of Handbookof PhysicalConstants [74], two othercollations Magnetic susceptibilityis a measureof the magnetic have appeared[16, 18]. In addition,selected magnetic responseof a materialto an externalmagnetic field. The parametershave alsobeen assembled[19, 22, 38, 41, 88]. volumesusceptibility k, measuredin dimensionlessunits, is Ratherthan produce a fully comprehensivecollection, we definedas the ratio of thematerial magnetization J (perunit have aimed for high-precisiondata obtainedfrom well- volume) to the weak externalmagnetic field H: characterizedsamples. Bothtables and figures have been used for presentingthe J=kH. (1) data,and best-fit equations have been provided for someof thedisplayed data so that interpolations can be made easily. Alternatively,the specificor masssusceptibility Z, mea- In an attemptto discouragethe useof the outdatedcgs suredin units of rn3kg4, isdefined as the ratio of the material system,all valuesare in theSI system(see Moskowitz, this magnetizationJ (per unit mass)to the weak externalmag- volume). Referenceshave been cited for the sourcesused netic field H: here. However,a morecomprehensive bibliography has alsobeen provided from which