Download Complete Work
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Family Descriptions
FAMILY DESCRIPTIONS CAT = Although they do not contain keys, the identification references include recent cata- logues as valuable source on genera, species, distribution and references. CMPD = Contributions to a Manual of Palaearctic Diptera. Lindner = Chapter in Lindner, E., Die Fliegen der Paläarktischen Region. ( ) Family names between brackets refer to names as found in the literature, not recognised here as a separate family but, as indicated, considered part of another family. et al. References with more than two authors are given as First author et al. As far as not yet outdated, the number of genera and species in Europe is largely based on the Catalogue of Palaearctic Diptera, the CMPD and Fauna Europaea, the latter available online at: www.faunaeur.org (consulted was version 1.2, updated 7 March 2005). As to size, the following categories are distinguished: minute: smaller than 2 mm; small: 2- 5 mm; medium sized: 5-10 mm; large: 10-20 mm; very large: over 20 mm. Acartophthalmidae (key couplet 113; fig. 243) Systematics: Acalyptrate Brachycera; superfamily Opomyzoidea; in Europe 1 genus, Acartophthalmus, with 3 species. Characters: Minute to small (1-2.5 mm), brownish grey flies. Arista pubescent, ocelli present; Oc-bristles present; P-bris- tles strong, far apart, diverging; 3 pairs of F-bristles, curving obliquely out-backward, increasing in size, the upper pair the largest; scattered interfrontal setulae present; vibrissae absent but with a series of strong bristles near the vibrissal angle. Wing unmarked or tinged along costa; costa with a humeral break only; vein Sc complete; crossvein BM-Cu present; cell cup closed. -

Some Type Specimens of Philippine Diptera Described by M
Pacific Insects ll (1) : 165-173 20 February 1969 SOME TYPE SPECIMENS OF PHILIPPINE DIPTERA DESCRIBED BY M. BEZZI IN THE MUSEO CIVICO DI STORIA NATURALE, MILANO1 By Mercedes D. Delfinado2 Abstract: The Bezzi Diptera collection now in the Museo Civico di Storia Naturale, Milano, contains the original series of many of the species from the Philippines collect ed by C. F. Baker, and lectotypes have been selected from these. This paper lists the type specimens of Philippine Diptera described by M. Bezzi as follows : Tipulidae : Eriocera crassipes, Libnotes marginalis, L. opaca, Pselliophora suspirans var. hilaris, P. tripudians, Trentepholia pictipennis; Rhagionidae: Chrysopilus diplostigma, Atherix fascipennis', Asilidae: Saropogon rubricosus, S. specularis; Bombyliidae: Sy str opus valdezi; Conopidae : Stylogaster bakeri; Neriidae : Telostylus niger; Nothybidae : Nothybus triguttatus; Platystomatidae: Euprosopia gigas, E. lepidophora, E. longicornis, E. millepunc- tata, E. trivittata, Loxoneura decora var. bakeri, Naupoda unifasciata, Pterogenia centralis, P. laticeps, P. luteipennis, P. parva, P. tristis, P. valida, Rivellia hendeliana, Scotinosoma ty- picum, Xenaspis extranea; Pyrgotidae : Campylocera thoracalis var. rufina; Lauxaniidae : Pachycerina apicalis, Steganopsis bakeri, Trigonometopus albiseta, T. bakeri; Lonchaeidae: Lonchaea calva, L. citricola, L. ficiperda, L. filifera; Chloropidae: Chromatopterum elegans, Tylopterna monstrosum; Cryptochaetidae : Cryptochaetum fastidiosum. Names are arranged alphabetically within the family and are listed under the original generic combinations. While preparing the Philippine Diptera catalog, it has been possible to check the species described by Mario Bezzi and to determine whether or not a type has been designated. In his "Studies in Philippine Diptera, I and II" the original description does not state the number of specimens in the original series and no type material was mentioned or des ignated. -

Chapter 2 Diopsoidea
Chapter 2 Diopsoidea DiopsoideaTeaching material only, not intended for wider circulation. [email protected] 2:37 Diptera: Acalyptrates DIOPSOI D EA 50: Tanypezidae 53 ------ Base of tarsomere 1 of hind tarsus very slightly projecting ventrally; male with small stout black setae on hind trochanter and posterior base of hind femur. Postocellar bristles strong, at least half as long as upper orbital seta; one dorsocentral and three orbital setae present Tanypeza ----------------------------------------- 55 2 spp.; Maine to Alberta and Georgia; Steyskal 1965 ---------- Base of tarsomere 1 of hind tarsus strongly projecting ventrally, about twice as deep as remainder of tarsomere 1 (Fig. 3); male without special setae on hind trochanter and hind femur. Postocellar bristles weak, less than half as long as upper orbital bristle; one to three dor socentral and zero to two orbital bristles present non-British ------------------------------------------ 54 54 ------ Only one orbital bristle present, situated at top of head; one dorsocentral bristle present --------------------- Scipopeza Enderlein Neotropical ---------- Two or three each of orbital and dorsocentral bristles present ---------------------Neotanypeza Hendel Neotropical Tanypeza Fallén, 1820 One species 55 ------ A black species with a silvery patch on the vertex and each side of front of frons. Tho- rax with notopleural depression silvery and pleurae with silvery patches. Palpi black, prominent and flat. Ocellar bristles small; two pairs of fronto orbital bristles; only one (outer) pair of vertical bristles. Frons slightly narrower in the male than in the female, but not with eyes almost touching). Four scutellar, no sternopleural, two postalar and one supra-alar bristles; (the anterior supra-alar bristle not present). Wings with upcurved discal cell (11) as in members of the Micropezidae. -

Diptera: Psilidae)
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 113: 393–396, 2016 http://www.eje.cz doi: 10.14411/eje.2016.050 NOTE Infestation of the mycoheterotrophic orchid Yoania japonica by the two-winged fl y, Chyliza vittata (Diptera: Psilidae) KENJI SUETSUGU Department of Biology, Graduate School of Science, Kobe University, 1-1 Rokkodai, Nada-ku, Kobe, 657-8501, Japan; e-mail: [email protected] Key words. Diptera, Psilidae, Chyliza vittata, host plants, mycoheterotrophy, Orchidaceae, Yoania japonica, Gastrodia elata, phytophagous insects, stem-miner Abstract. Chyliza vittata is known to utilize leaves, stems and underground parts of several leafy and leafl ess orchids. Compared to the well-recorded feeding habits of C. vittata in Europe, its feeding habits in Japan are poorly studied. Thus, further records of its host plants and the habits of its larvae in Japan are likely to reveal the similarities and differences in its feeding habits in Europe and Japan. The current study reports C. vittata feeding on the stems of the mycoheterotrophic orchid Yoania japonica in central Japan. This study also showed that in spite of the small size of Yoania its reproductive success is not severely reduced when infested with C. vittata, whereas the robust stems of Gastrodia elata, which is its main host plant in Japan, are thought to be a defence against infestation by C. vittata. INTRODUCTION fl oribunda (Caprifoliaceae; Sugiura & Yamazaki, 2006; Yamaza- The Psilidae is a small family of acalyptrate Diptera in the su- ki & Sugiura, 2008), while C. vittata consumes the leaves, stems perfamily Diopsoidea, which includes about 400 described spe- and underground structures of several orchid genera, including cies (Freidberg & Shatalkin, 2008). -
A Commentary on the Practice of Using the So-Called Typeless Species
A peer-reviewed open-access journal ZooKeys 693: 129–139 (2017)A commentary on the practice of using the so-called typeless species 129 doi: 10.3897/zookeys.693.10945 SHORT COMMUNICATION http://zookeys.pensoft.net Launched to accelerate biodiversity research A commentary on the practice of using the so-called typeless species Anatoly I. Shatalkin1, Tatiana V. Galinskaya2 1 Zoological Museum, Lomonosov Moscow State University, 6 Bol’shaya Nikitskaya St., Moscow, 125009, Russia 2 Lomonosov Moscow State University, Biological faculty, Entomology department, Leninskie gory 1-12, Moscow, 119234, Russia Corresponding author: Tatiana V. Galinskaya ([email protected]) Academic editor: A. Minelli | Received 27 October 2016 | Accepted 2 March 2017 | Published 23 August 2017 http://zoobank.org/AAD7E722-4775-43D2-94F0-209C3A75B74F Citation: Shatalkin AI, Galinskaya TV (2017) A commentary on the practice of using the so-called typeless species. ZooKeys 693: 129–139. https://doi.org/10.3897/zookeys.693.10945 Abstract The fears expressed by Santos et al. (2016) that description of typeless species (new species described based on field photographs) can be fatal for the practice of taxonomy which will succumb to an uncontrollable stream of “species of questionable delimitation” are, in our opinion, exaggerated. The Code already pro- tects taxonomic practice from subjectivity quite well by limiting opportunities for descriptions of new spe- cies based on field photos by rigid requirements, and only skilled taxonomists with extensive knowledge of a group are capable of fulfilling them. If a taxonomist has omitted to compare the new typeless species with the known species externally similar to it, the latter cannot be diagnosed and its name in that case becomes nomen nudum. -

Proceedings of the United States National Museum
REVISION OF THE TWO-WINGED FLIES OF THE FAMILY CLUSIIDAE. By A. L. Melander and Naomi George Argo, Of the State College of Washington, Pullman. The family Clusiidae, sometimes called the Heteroneuridae or the Clusiodidae, is generally regarded as one of the rarer groups of the Diptera. Seldom are its members met with in more than solitary individuals. In our experience in collecting a hundred thousand specimens of Diptera but a few dozen representatives of Clusiidae have been encountered. Previously there have been described from the entire world 13 valid genera and 55 species. Aldrich's Catalogue in 1905 listed but 2 genera and 12 species as known from North America. The sub- sequent publications of Johnson and Malloch have added 11 recog- nized new species to the American list. Fifteen species have been described from Europe and the same number from South America, while four species have been recorded from the islands south of Asia. The material secured for the present study, amounting to some 400 specimens, has produced 52 species, of which 25 are new. With the extension in distribution of species originally described from Europe or South America there are now known to occur in North America, including Central America, a total of 58 species belonging to 7 genera. Thus in its present status the family includes 80 recognized species distributed among 13 genera. The Clusiidae are restricted in their distribution to Europe, North and South America, and the East Indies. No species have been described from Africa, Australia, or Asia, but there is mention by Lefroy of the occurrence of an undetermined species in India. -
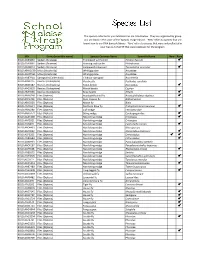
Species List
The species collected in your Malaise trap are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in your trap out of all 59 that were deployed for the program. BIN Group (scientific name) Species Common Name Scientific Name New Rare BOLD:AAB3890 Spiders (Araneae) Thinlegged wolf spider Pardosa fuscula BOLD:AAA9991 Spiders (Araneae) Running crab spider Philodromus BOLD:AAB8024 Spiders (Araneae) Longjawed orbweaver Tetragnatha versicolor BOLD:ABW2639 Mites (Arachnida) Whirligig mite Anystidae BOLD:AAM7961 Mites (Arachnida) Whirligig mite Anystidae BOLD:AAB7914 Springtails (Collembola) Globular springtail Bourletiella BOLD:ABX3225 Beetles (Coleoptera) Flea beetle Psylliodes cucullata BOLD:ABA0187 Beetles (Coleoptera) Snout beetle Dorytomus BOLD:AAG3633 Beetles (Coleoptera) Marsh beetle Cyphon BOLD:ABA9956 Beetles (Coleoptera) Rove beetle Atheta BOLD:AAN6240 Flies (Diptera) Acartophthalmid fly Acartophthalmus nigrinus BOLD:ACP6334 Flies (Diptera) Root maggot fly Anthomyiinae BOLD:ABV0350 Flies (Diptera) March fly Bibio BOLD:AAC9614 Flies (Diptera) Northern blow fly Protophormia terraenovae BOLD:ABX0280 Flies (Diptera) Gall midge Cecidomyiidae BOLD:AAN5172 Flies (Diptera) Biting midge Ceratopogonidae BOLD:AAA5300 Flies (Diptera) Non-biting midge Cricotopus BOLD:AAP5921 Flies (Diptera) Non-biting midge Cricotopus BOLD:AAI9470 Flies (Diptera) Non-biting midge Cyphomella -
X-Ray Microtomography (Microct) of Male Genitalia of Nothybus Kuznetsovorum (Nothybidae) and Cothornobata Sp
A peer-reviewed open-access journal ZooKeys 744:X-ray 139–147 microtomography (2018) (microCT) of male genitalia of Nothybus kuznetsovorum... 139 doi: 10.3897/zookeys.744.22347 SHORT COMMUNICATION http://zookeys.pensoft.net Launched to accelerate biodiversity research X-ray microtomography (microCT) of male genitalia of Nothybus kuznetsovorum (Nothybidae) and Cothornobata sp. (Micropezidae) Tatiana V. Galinskaya1,2, Dina Gafurova (Gilyazetdinova)3, Olga G. Ovtshinnikova4 1 Faculty of Biology, Lomonosov Moscow State University, Moscow, 119234 Russia 2 Museum of Entomology, All-Russian Plant Quarantine Center, Pogranichnaya 32, Bykovo, 140150, Russia 3 Faculty of Geology, Lomo- nosov Moscow State University, Moscow, 119234 Russia 4 Zoological Institute, Russian Academy of Sciences, St. Petersburg, 199034 Russia Corresponding author: Tatiana V. Galinskaya ([email protected]) Academic editor: M. Hauser | Received 17 November 2017 | Accepted 27 February 2018 | Published 20 March 2018 http://zoobank.org/751CAAB3-6BC5-46AD-9631-94E44109D7DA Citation: Galinskaya TV, Gafurova (Gilyazetdinova) D, Ovtshinnikova OG (2018) X-ray microtomography (microCT) of male genitalia of Nothybus kuznetsovorum (Nothybidae) and Cothornobata sp. (Micropezidae). ZooKeys 744: 139–147. https://doi.org/10.3897/zookeys.744.22347 Abstract The results of manual dissection of the musculature of the male genitalia in Nothybus kuznetsovorum are fully confirmed by the modern methods of Micro-CT. A comparative analysis of Neria commutata and Cothornobata sp. shows that an increase in the flexion in the genitalia of males and the displacement of syntergosternite VII to the ventral side in Cothornobata sp. caused the disappearance of the muscles ITM6–7r and ITM7–8r. In addition, this increase in flexion apparently caused the fusion of the M18 muscles into one bundle. -

Diptera. Benno Wandolleck
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Archiv für Naturgeschichte Jahr/Year: 1897 Band/Volume: 63-2_2 Autor(en)/Author(s): Wandolleck Benno Artikel/Article: Diptera. 289-346 © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Diptera. Bearbeitet von Dr. Benno Wandolleek. Aldrich, J. M. The Dipterous genera Tachytrechus and Macello- cerns. Tr. Amer. ent. Soc. XXIII, p. 81—84. Alessandrill t, (x. Un caso di myiasis per larve della Sarcophaga carnaria Meig. Boll. Soc. Rom. Zool. v., p. 194— 197. Arkle, J. The senses of insects. Entomologist 1896, p. 343— 345. Austen, E. E. (1). Necrophagous Diptera attracted by the odoiir of flowers. Ann. Nat. Eist. (6) XVIII, p. 237—240. Verf. erhielt von dem Vorsteher des Botanical Department in Trinidad eine Kollektion von Dipteren, die in einer Blume von Aristolochia f/ic/as var. startevcmtii gefangen waren. Die Blüthe dieser Pflanze hat einen starken Fleischgeruch, sodass sie sogar schon Geier angezogen hat. Da die Thiere nicht sehr gut erhalten waren, konnten sie nicht genau bestimmt werden. Es waren: 2 verschiedene Lucilia sp. ungefähr je 6 Exemplare, Conipromyia macellaria F. 12 Exemplare. 1 Tachinc gen. et sp. incert, 1 $ von Musca domestica L., 2 (^ 2 $ einer Sarcophaga sp., 1 (^ 6 $ von Ophyra aenescens Wiedem. Musca domestica war bis dahin nicht von Trinidad bekannt. Verf. macht noch einige biologische Be- merkungen über das Vorkommen von Dipteren in stark riechenden Blüthen. — (2). Notes on a recent zoological expedition on the Lower Amazon. P. Zool. Soc. London. 1896, p. 768—779. -

Torngat Mountains Taxonomy Report
Torngat Mountains Taxonomy Report Class Order Family Species Arachnida Araneae Linyphiidae Praestigia kulczynskii Mesostigmata Laelapidae Pachylaelapidae Insecta Lepidoptera Argyresthiidae Argyresthia pygmaeella Coleophoridae Coleophora canadensisella Crambidae Gelechiidae Geometridae Gracillariidae Acrocercops astericola Parornix obliterella Phyllonorycter anderidae Phyllonorycter hilarella Incurvariidae Incurvaria circulella Momphidae Nepticulidae Stigmella betulicola Tortricidae Yponomeutidae Paraswammerdamia lapponica Coleoptera Chrysomelidae Elateridae Staphylinidae Diptera Acartophthalmidae Acartophthalmus nigrinus Agromyzidae Chromatomyia opacella Anisopodidae Sylvicola fuscatus Anthomyiidae Alliopsis benanderi Delia longicauda Pegomya circumpolaris Pegomya icterica Pegoplata tundrica Anthomyzidae Borboropsidae Borboropsis puberula Brachystomatidae Heleodromia pullata Calliphoridae Cynomya cadaverina Cecidomyiidae Ceratopogonidae Chaoboridae Chironomidae Ablabesmyia monilis Chironomus longistylus Cladotanytarsus atridorsum Halocladius variabilis Krenosmittia halvorseni Limnophyes brachytomus Limnophyes schnelli Metriocnemus eurynotus Micropsectra logani Micropsectra polita Parakiefferiella scandica Parametriocnemus boreoalpinus 1 Paratanytarsus austriacus Smittia aterrima Tanytarsus gregarius Tvetenia paucunca Chloropidae Thaumatomyia trifasciata Culicidae Cylindrotomidae Dolichopodidae Dolichopus brevipennis Dolichopus groenlandicus Dolichopus nigrilineatus Scellus amplus Empididae Dolichocephala chillcotti Fanniidae Fannia atripes -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Diptera: Diopsoidea), and a Discussion of Relationships of the Diopsoid Families
Records of the Australian Museum (1997) Vol. 49: 167-194. ISSN 0067-1975 Gobryidae, aNew Family of Acalyptrate Flies (Diptera: Diopsoidea), and a Discussion of Relationships of the Diopsoid Families DAVID K. McALPINE Australian Museum, 6 College Street, Sydney NSW 2000, Australia ABSTRACT. Relationships among families referred to the superfamily Diopsoidea (or Nothyboidea) are discussed from the evidence of comparative morphology, particular attention being given to the Nothybidae, Psilidae, Syringogastridae, and Diopsidae. Some comments are made on selection of autapomorphies in cladistic methodology. The Tanypezidae and Somatiidae are removed from the Diopsoidea to incertae sedis. The new diopsoid family Gobryidae, or hinge flies, is established for the Oriental-Australasian genus Gobrya Walker, previously variously associated with the families Megamerinidae, Nothybidae, and Syringogastridae. A key to the families which have been included in Diopsoidea is given. A systematic arrangement of taxa mentioned in the discussion is appended. MCALPlNE, DAVID K., 1997. Gobryidae, a new family of acalyptrate flies (Diptera: Diopsoidea), and a discussion of relationships of the diopsoid families. Records of the Australian Museum 49(2): 167-194. The genus Gobrya was described by Walker (1860) McAlpine (1997) transferred the Megamerinidae, in the "Subfam. Psilides" of the family Muscidae. excluding Gobrya, to the Nerioidea. Walker's only originally included species of Gobrya Gobrya is known from Taiwan, the Philippines, was G. bacchoides Walker, from Celebes, Indonesia Malaysia, Indonesia, and New Guinea (Steyskal, 1977; (type species by monotypy). and author's observations). Steyskal listed five described Hendel (1913) treated Gobrya and his newly described species of Gobrya (loc. cit.), but has generously genus Syritfomyia in the "Megamerininae", later raised supplied me with a copy (dated 1967) of a preliminary to family rank as Megamerinidae.