Second Dose Considerations for Astrazeneca/COVISHIELD First Dose
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ifs Recommendations for Covid 19 Vaccination Before Art
16 – 17 JULY 2020 PRAGATI MAIDAN - DELHI INDIAN FERTILITY SOCIETY IFS RECOMMENDATIONS FOR COVID 19 VACCINATION BEFORE ART IFS SECRETARIAT +91 11 40018184 +91 9899308083 indianferlitysociety [email protected] www.indianferlitysociety.org indianferlitysociety 302, 3rd Floor, Kailash Building, ifsdelhi 26, Kasturba Gandhi Marg, C.P. New Delhi - 110001 Cosmo Tech is the Most Important Trade show for the Suppliers to EXPAND Strengthen Increase Multiply Profits New Markets Pan New Market Customer Base India & World Customer Soaring Boost Network Brand Sales Brand Recall Value with the Industry Visibility ORGANIZED BY CALL EMAIL +91 9971811937 [email protected] +91 9999302797 [email protected] +91 9811141938 [email protected] W W W .C O SMO TEC HEXPO IND IA .C O M Dr. Sudha Prasad Dr. Neena Malhotra President Secretary General Indian Fertility Society Indian Fertility Society Director, Matritava Advanced IVF & Professor, ART Centre, Department of Training Cenre, Vasant Vihar, New Delhi Obstetrics and Gynaecology, AIIMS, New Delhi Dr. Sonia Malik Dr. Kuldeep Jain Past President Indian Fertility Society, Past President Indian Fertility Society, Director & Nova Southend Fertility & Director, KJIVF New Delhi IVF Delhi NCR Dr. KU Kunjumoideen Dr. A K Pandey Joint Secretary Indian Fertility Society, Dean Academics &. Co Ordinator Director ARMC IVF Calicut. Molecular Lab. ESI Medical college Faridabad Dr. Charu Jandial Dr. Sumita Aggarwal Member IFS, Consultant, Member IFS, Fellow, Nova Southend Fertility & IVF Delhi NCR Nova Southend Fertility & IVF Delhi NCR Introduction The coronavirus pandemic has wreaked havoc on life In these trying mes, as sociees are gradually trying to and healthcare globally. According to WHO database as return to a state of normalcy, it is also important to on 7th June 2021, there have been 173 million consider sexual and reproducve health of people. -

The 6-In-1 Vaccine Study
Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk The 6-in-1 vaccine study Study Information Booklet We are inviting infants aged 8-13 weeks of age to take part in a study comparing two licensed combination vaccines that can be given in the routine UK immunisation schedule. These vaccines protect against 6 different infections in the one injection. Before you decide to take part in this study, it is important for you to understand what the study is about and what participation would involve. Please take time to read the information carefully, and discuss with others if you wish. If you have any questions please contact the study team. Thank you for taking the time to consider this study. Contact the local study team at: Oxford Vaccine Group CCVTM, Churchill Hospital, Oxford, OX3 7LE 01865 611400 [email protected] www.ovg.ox.ac.uk/recruiting-studies Page 1 of 11 The 6-in-1 vaccine study; OVG 2018/05; Participant Information Booklet; REC Ref: 19/SC/0052; IRAS ID: 252324; Version 2.0 dated 23-APR-2019 Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk Summary • This study will help us to better understand the interaction between ‘6- in-1’ vaccines and the Meningococcal B vaccine in the routine UK immunisation schedule • We will be enrolling 240 healthy babies aged 8 – 13 weeks into this study • Babies will receive all their infant immunisations in their own home or in a convenient location until 12 months of age • There will be six study visits including 4 vaccine visits and 2 visits for blood sampling (we will use a topical anaesthetic cream to numb the skin for blood sampling) Why has my child been invited to take part? You have been approached as your child is in the age range for this study and lives in the Thames Valley. -

Review of COVID-19 Vaccine Subtypes, Efficacy and Geographical Distributions Andre Ian Francis ,1 Saudah Ghany,1 Tia Gilkes,1 Srikanth Umakanthan2
Review Postgrad Med J: first published as 10.1136/postgradmedj-2021-140654 on 6 August 2021. Downloaded from Review of COVID-19 vaccine subtypes, efficacy and geographical distributions Andre Ian Francis ,1 Saudah Ghany,1 Tia Gilkes,1 Srikanth Umakanthan2 1Department of Clinical Medical ABSTRACT 2021, the Ad26.COV2.S, developed by Janssen Sciences, The Faculty of Medical As of 1 May 2021, there have been 152 661 445 (Johnson & Johnson) and Moderna on 30 April.4 Sciences, The University of the COVAX, coordinated by WHO, Gavi: The West Indies, St. Augustine, Covid-19 cases with 3 202 256 deaths globally. Trinidad and Tobago This pandemic led to the race to discover a vaccine Vaccine Alliance, the Coalition for Epidemic 2Pathology unit, Department to achieve herd immunity and curtail the damaging Preparedness Innovations (CEPI), acts as a of Paraclinical Sciences, The effects of Covid-19. This study aims to discuss the most programme that supports the development of University Of The West Indies, St. COVID-19 vaccine candidates and negotiates their Augustine, Trinidad and Tobago recent WHO-approved Covid-19 vaccine subtypes, their status and geographical scheduled updates as of 4 pricing to ensure low- and- middle- income countries have a fair shot at receiving vaccines.5 Correspondence to May 2021. The keywords “Covid-19, Vaccines, Pfizer, Mr. Andre Ian Francis, The BNT162b2, AstraZeneca, AZD1222, Moderna, mRNA- This article aims at discussing the most recent University of the West Indies, St. 1273, Janssen, Ad26.COV2.S” were typed into PubMed. WHO- approved COVID-19 vaccine subtypes, their Augustine, Trinidad and Tobago; Thirty Two relevant PubMed articles were included in status and geographical scheduled updates as of 4 andre. -
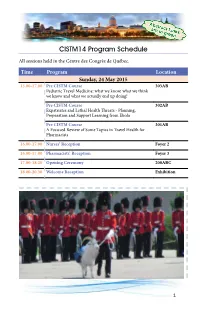
CISTM14 Program Schedule
CISTM14 Program Schedule All sessions held in the Centre des Congrès de Québec. Time Program Location Sunday, 24 May 2015 13.00-17.00 Pre CISTM Course 303AB Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! Pre CISTM Course 302AB Expatriates and Lethal Health Threats - Planning, Preparation and Support Learning from Ebola Pre CISTM Course 301AB A Focused Review of Some Topics in Travel Health for Pharmacists 16.00-17.00 Nurses’ Reception Foyer 2 16.00-17.00 Pharmacists’ Reception Foyer 3 17.00-18.20 Opening Ceremony 200ABC 18.00-20.30 Welcome Reception Exhibition 1 Time Program Location Monday, 25 May 2015 MTH1 Meet The History 301AB 8.00-8.45 History of the Quarantine Station at Grosse Ile Marc Desmeules, Canada 8.00-8.45 CDC Yellow Book 302AB Gary Brunette, United States of America COD1 Case of the Day 303AB 8.00-8.45 Pre-Travel Rogelio Lopez-Velez, Spain PL1 Plenary 200ABC 9.00-10.30 Our Shrinking World: Health in the 21st Century Chairs: David R. Shlim, United States of America Leo Visser, The Netherlands PL1.01 Measuring global health: The global is local [Change your mindset! It’s time for a reality check] Louis Loutan, Switzerland • Outline the most important trends and health challenges in the world using gapminder/health metrics • Appraise how these changes (global health transitions, global population growth, urbanization, changes in animal helath and human-animal population interactions, and climate change) will influence global migration and travel medicine now and in the future • Integrate global health concepts into thinking about travel medicine 2 Time Program Location Monday, 25 May 2015, continued Cont. -

Different Types of COVID-19 Vaccines
Pfizer-BioNTech Help stop the pandemic Type of vaccine: Messenger RNA, or mRNA, a genetic Different Types material that tells your body how to make proteins that by getting vaccinated triggers an immune response inside our bodies of COVID-19 Effectiveness: 95% based on clinical trials Even if you are undocumented Common side effects: Pain and/or swelling in the arm and/or don’t have insurance, you and tiredness, headache, muscle pain, chills, fever, or can get the vaccine—for free. Vaccines: nausea in the body that may last two days Recommended Ages: 16 and older (currently testing the vaccine in kids ages 12-15) Understanding How Dosage: Two shots, 21 days apart They Work Visit VaccinateALL58.com Moderna for the newest information about when and where the vaccine Type of vaccine: Messenger RNA, or mRNA, a genetic will be available to you. material that tells your body how to make proteins that triggers an immune response inside our bodies Sign up at myturn.ca.gov or Effectiveness: 94.1% based on clinical trials call 1-833-422-4255 to find out Common side effects: Pain and/or swelling in the arm if it’s your turn to get vaccinated and and tiredness, headache, muscle pain, chills, fever, or schedule vaccination appointments. nausea in the body that may last two days Recommended Ages: 18 years and older (currently testing the vaccine in kids ages 12-17) Dosage: Two shots, 28 days apart Johnson & Johnson Follow us on social media for more COVID-19 tips and information. Type of vaccine: A viral vector, it uses a harmless version of a different -

Sir Bryn Terfel Premieres John Rutter's 'Joseph's Carol', Dedicated to the Oxford Vaccine Team in Celebratory Concert Fr
Sir Bryn Terfel premieres John Rutter’s ‘Joseph’s Carol’, dedicated to the Oxford vaccine team in celebratory concert from the Oxford Philharmonic Orchestra Friday 18 December 2020, 18:30 Streamed on Oxford Philharmonic Orchestra’s YouTube Channel: bit.ly/OPOVaccineTribute Elgar Chanson de Matin William Henry Monk Abide with Me Rodgers & Hammerstein You’ll Never Walk Alone John Rutter Joseph’s Carol WORLD PREMIERE John Rutter Look to the Day Handel Hallelujah Chorus Sir Bryn Terfel bass-baritone Oxford Philharmonic Orchestra Maxim Vengerov violin Choir of Merton College, Oxford John Rutter conductor Alexandra Lowe soprano Marios Papadopoulos conductor Alexander Olleson treble John Suchet presenter In recognition of the formidable work accomplished by the team of scientists at the University of Oxford on their Covid-19 vaccine, the Oxford Philharmonic Orchestra will stream a celebratory concert on Friday 18 December, recorded in the city’s historic Sheldonian Theatre. Performed by bass-baritone Sir Bryn Terfel, the short concert features the premiere of John Rutter’s Joseph’s Carol, written in tribute to the Oxford Vaccine Group, the Jenner Institute and the RECOVERY team. The words by John Rutter recount the long and weary journey of Joseph and Mary to Bethlehem before the birth of the baby Jesus, echoing the programme’s journey from struggle through to hope. Bryn Terfel also joins the Orchestra and the Choir of Merton College, Oxford, in a rousing programme from Rodgers & Hammerstein’s You’ll Never Walk Alone (with Jette Parker Young Artist Alexandra Lowe) to Handel’s Hallelujah Chorus. Sir Bryn and the Orchestra are also joined in the hymn of comfort, Abide with Me, by chorister Alexander Olleson of Christ Church Cathedral Choir, the recent winner of BBC Young Chorister Of The Year 2020. -

Adenoviral Vector COVID-19 Vaccines: Process and Cost Analysis
processes Article Adenoviral Vector COVID-19 Vaccines: Process and Cost Analysis Rafael G. Ferreira 1,* , Neal F. Gordon 2, Rick Stock 2 and Demetri Petrides 3 1 Intelligen Brasil, Sao Paulo 01227-200, Brazil 2 BDO USA, LLP, Boston, MA 02110, USA; [email protected] (N.F.G.); [email protected] (R.S.) 3 Intelligen, Inc., Scotch Plains, NJ 07076, USA; [email protected] * Correspondence: [email protected] Abstract: The COVID-19 pandemic has motivated the rapid development of numerous vaccines that have proven effective against SARS-CoV-2. Several of these successful vaccines are based on the adenoviral vector platform. The mass manufacturing of these vaccines poses great challenges, especially in the context of a pandemic where extremely large quantities must be produced quickly at an affordable cost. In this work, two baseline processes for the production of a COVID-19 adenoviral vector vaccine, B1 and P1, were designed, simulated and economically evaluated with the aid of the software SuperPro Designer. B1 used a batch cell culture viral production step, with a viral titer of 5 × 1010 viral particles (VP)/mL in both stainless-steel and disposable equipment. P1 used a perfusion cell culture viral production step, with a viral titer of 1 × 1012 VP/mL in exclusively disposable equipment. Both processes were sized to produce 400 M/yr vaccine doses. P1 led to a smaller cost per dose than B1 ($0.15 vs. $0.23) and required a much smaller capital investment ($126 M vs. $299 M). The media and facility-dependent expenses were found to be the main contributors to the operating cost. -

An Overview of COVID Vaccine Clinical Trial Results & Some Challenges
An overview of COVID vaccine clinical trial results & some challenges DCVMN Webinar December 8th, 2020 Access to COVID-19 tools ACCESSACCESS TO TOCOVID-19 COVID-19 TOOLS TOOLS (ACT) (ACT) ACCELERATOR ACCELERATOR (ACT) accelerator A GlobalA GlobalCollaboration Collaboration to Accelerate tothe AccelerateDevelopment, the Production Development, and Equitable Production Access to New and Equitable AccessCOVID-19 to New diagnostics, COVID-19 therapeutics diagnostics, and vaccines therapeutics and vaccines VACCINES DIAGNOSTICS THERAPEUTICS (COVAX) Development & Manufacturing Led by CEPI, with industry Procurement and delivery at scale Led by Gavi Policy and allocation Led by WHO Key players SOURCE: (ACT) ACCELERATOR Commitment and Call to Action 24th April 2020 ACT-A / COVAX governance COVAX COORDINATION MEETING CEPI Board Co-Chair: Jane Halton Co-Chair: Dr. Ngozi Gavi Board Workstream leads + DCVMN and IFPMA-selected Reps As needed – R&D&M Chair; COVAX IPG Chair Development & Manufacturing Procurement and delivery Policy and allocation (COVAX) at scale Led by (with industry) Led by Led by R&D&M Investment Committee COVAX Independent Product Group Technical Review Group Portfolio Group Vaccine Teams SWAT teams RAG 3 COVAX SWAT teams are being set up as a joint platform to accelerate COVID- 19 Vaccine development and manufacturing by addressing common challenges together Timely and targeted Multilateral Knowledge-based Resource-efficient Addresses specific cross- Establishes a dialogue Identifies and collates Coordinates between developer technical and global joint effort most relevant materials different organizations/ challenges as they are across different COVID-19 and insights across the initiatives to limit raised and/or identified vaccines organizations broader COVID-19 duplications and ensure on an ongoing basis (incl. -

Update 50 (15Th of December 2020)
Update 50 (15th of December 2020) Information about Infection disease COVID-19 (novel coronavirus) Force Health Protection Branch FHPB (former DHSC) NATO MILMED COE in Munich 15th of December 2020 email: [email protected] In December 2019, a novel coronavirus emerged in Wuhan City, China. Since then the virus spread to 65 countries including Europe and America. Since then the virus showed evidence for human-to-human transmission as well as evidence of asymptomatic transmission. At 30th January 2020 WHO declared a Public Health Emergency of International Concern. The disease was formally named COVID-19 on 11th of February. The virus itself has been named SARS-CoV-2. On 11th of March 2020 WHO characterized the disease as a pandemic. HIGHLIGHTS/NEWS GLOBALLY ↗ • GBR/WHO: A new variant of the virus could be responsible for the renewed high number of cases in the UK, among other things, which 72 879 777 confirmed cases could be "in connection with the faster spread in the south of England". 47 710 950 recovered So far, 1000 cases have been identified. 1 622 200 deaths The WHO emphasized that the new variant would now be examined. EU/EEA and the UK ↘ However, there is no evidence that the strain behaves differently than 21 936 276 existing virus types. "We saw many variants, this virus evolves and confirmed cases changes over time." 10 689 950 recovered • Sputnik V: The developers of the Russian coronavirus vaccine Sputnik 479 566 deaths V explain that it creates 91.4 percent protection against the lung disease USA ↗ Covid-19. -

Gene-Based Vaccines (GBV)
Advanced Drug Delivery Reviews 170 (2021) 113–141 Contents lists available at ScienceDirect Advanced Drug Delivery Reviews journal homepage: www.elsevier.com/locate/addr Advances in gene-based vaccine platforms to address the COVID-19 pandemic Deborah Pushparajah a,1, Salma Jimenez a,c,1,ShirleyWonga,HibahAlattasa, Nafiseh Nafissi b, Roderick A. Slavcev a,b,c,⁎ a School of Pharmacy, University of Waterloo, 10A Victoria St S, Kitchener N2G 1C5, Canada b Mediphage Bioceuticals, 661 University Avenue, Suite 1300, Toronto, ON, M5G 0B7, Canada c Theraphage, 151 Charles St W Suite # 199, Kitchener, ON, N2G 1H6, Canada article info abstract Article history: The novel betacoronavirus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has spread across Received 1 October 2020 the globe at an unprecedented rate since its first emergence in Wuhan City, China in December 2019. Scientific Received in revised form 23 December 2020 communities around the world have been rigorously working to develop a potent vaccine to combat COVID-19 Accepted 1 January 2021 (coronavirus disease 2019), employing conventional and novel vaccine strategies. Gene-based vaccine platforms Available online 7 January 2021 based on viral vectors, DNA, and RNA, have shown promising results encompassing both humoral and cell-mediated immune responses in previous studies, supporting their implementation for COVID-19 vaccine de- Keywords: Coronavirus velopment. In fact, the U.S. Food and Drug Administration (FDA) recently authorized the emergency use of two COVID-19 RNA-based COVID-19 vaccines. We review current gene-based vaccine candidates proceeding through clinical SARS-CoV-2 trials, including their antigenic targets, delivery vehicles, and route of administration. -

Download a PDF of Our Community Brochure
Engagement with the communities of Oxford and Oxfordshire Did you know? St Giles’ Fair began as the parish feast of St Giles, first recorded in 1624. From the 1780s it became a toy fair, with general amusements for children. In the next century its focus shifted towards adults, with entertainment, rides and stalls. In the late 1800s there were calls for the fair to be stopped on the grounds that it encouraged rowdy behaviour. During Victorian times engineering advances brought the forerunners of today’s rides. Today the huge pieces of machinery fill St Giles’ with sparkling lights for a few days each year, and whizz within feet of ancient college buildings. The stone heads around the Sheldonian Theatre now number thirteen (there were originally fourteen, but one was removed to make way for the adjoining Clarendon Building.) It is not known what they were intended to represent – they might be gods, wise men, emperors or just boundary markers. The original heads were made by William Byrd and put up in 1669. Did you Replacements put up in 1868 were made in poor stone, know? which crumbled away; in 1972 the current set, carved by Michael Black of Oxford, were erected. More on page 4 STARGAZING AND SPIN-OUTS PAGE 1 Contents 2 Introduction from the Vice-Chancellor 3 Foreword from the Chair of the Community Engagement Group 5 Part 1: Part of the fabric of the city Part of the fabric 6 800 years of history of the 8 Economic impact city 9 Science Parks 1 0 Saïd Business School 11 Oxford University Press PART 1 PART 1 2 The built environment 13 -

RCN International Nursing Research Conference 2017
RCN International Nursing Research Conference 2017 Wednesday 5 – Friday 7 April 2017 University of Oxford Examination Schools, 75-81 High Street, Oxford, OX1 4BG, UK Conference abstracts media partner Accrue up to 27 hours #research2017 of CPD Contents Keynote speaker abstracts 4 Concurrent session 6 54 Thursday 6 April 2017 2-2.55pm 54 Theme: Focus groups .................................54 Concurrent session 1 6 Theme: Mixed ........................................55 Wednesday 5 April 2017 11.30am-12.55pm 6 Theme: Qualitative approaches/patient safety and experience .....................................56 Theme: Qualitative approaches ..........................6 Theme: Qualitative approaches/interviews ...............57 Theme: Qualitative approaches .......................... 7 Theme: Evidence review ...............................58 Theme: Qualitative approaches ..........................8 Theme: Qualitative approaches/text and discourse ........59 Theme: Evidence review/patient safety ..................10 Theme: Questionnaires/other methods ..................60 Theme: Mixed eHealth .................................11 Theme: Research methodology ......................... 13 Concurrent session 7 62 Theme: Mixed methods/patient experience ............... 14 Friday 7 April 2017 9.50-10.45am 62 Concurrent session 2 17 Theme: Workforce/review .............................62 Wednesday 5 April 2017 1.55-3.20pm 17 Theme: Qualitative approaches .........................63 Theme: Qualitative approaches/interviewing .............64 Theme: Qualitative