Cell Death Via Lipid Peroxidation and Protein Aggregation Diseases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Regulation of Mitochondrial and Nonmitochondrial Protein Turnover by the PINK1-Parkin Pathway
Regulation of mitochondrial and nonmitochondrial protein turnover by the PINK1-Parkin pathway Evelyn S. Vincow A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2013 Reading Committee: Leo J. Pallanck, Chair Sandra M. Bajjalieh Michael J. MacCoss Program Authorized to Offer Degree: Neurobiology and Behavior © Copyright 2013 Evelyn S. Vincow University of Washington Abstract Regulation of mitochondrial and nonmitochondrial protein turnover by the PINK1-Parkin pathway Evelyn Sandra Vincow Chair of the Supervisory Committee: Associate Professor Leo J. Pallanck Genome Sciences The accumulation of damaged mitochondria has been proposed as a key factor in aging and in the pathogenesis of many common age-related diseases, including Parkinson disease (PD). Recently, in vitro studies of the PD-related proteins Parkin and PINK1 have found that these factors act in a common pathway to promote the selective autophagic degradation of damaged mitochondria (mitophagy). However, whether PINK1 and Parkin promote mitophagy in vivo is unknown. To address this question, I used a proteomic approach in Drosophila to study the effects of null mutations in parkin or PINK1 on mitochondrial protein turnover. The parkin null mutants showed a significant overall slowing of mitochondrial protein turnover, similar to but less severe than the slowing seen in autophagy-deficient Atg7 mutants, consistent with the model that Parkin acts upstream of Atg7 to promote mitophagy. By contrast, the turnover of many mitochondrial respiratory chain (RC) subunits showed greater impairment in parkin than in Atg7 mutants, and RC turnover was also selectively impaired in PINK1 mutants. These findings demonstrate that the PINK1-Parkin pathway promotes mitophagy in vivo and, unexpectedly, also promotes selective turnover of mitochondrial RC components. -

An ACSL4 Hemizygous Intragenic Deletion in a Patient with Childhood Stroke
Pediatric Neurology 100 (2019) 100e101 Contents lists available at ScienceDirect Pediatric Neurology journal homepage: www.elsevier.com/locate/pnu Clinical Letter An ACSL4 Hemizygous Intragenic Deletion in a Patient With Childhood Stroke Caitlin A. Chang, MD a, Julie Lauzon, MD, MHSc a, b, Adam Kirton, MD, MSc b, c, * Bob Argiropoulos, PhD a, b, d, a Department of Medical Genetics, Alberta Children's Hospital, Calgary, Alberta, Canada b Alberta Children's Hospital Research Institute for Child and Maternal Health, Alberta Children's Hospital, Calgary, Alberta, Canada c Department of Pediatrics and Clinical Neurosciences, Pediatric Neurology, Alberta Children's Hospital, Calgary, Alberta, Canada d Genetic Laboratory Services, Cytogenetics Laboratory, Alberta Children's Hospital, Calgary, Alberta, Canada article info Article history: Received 4 March 2019 Accepted 22 June 2019 Available online 28 June 2019 Keywords: Childhood stroke ACSL4 X-linked intellectual disability Ischemia Developmental delay We describe a male with a maternally inherited 9.75-kb intra- was present. Additional evaluation for stroke risk factors, including genic deletion of ACSL4. Fewer than 25 individuals have been re- lumbar puncture, echocardiogram, and examination of blood for fl ported with ACSL4-related X-linked intellectual disability (XLID). thrombophilia and in ammatory markers were negative. This individual highlights a unique presentation of ACSL4-related At three years, severe communication delays were evident. fi XLID and reveals additional pathways for investigation. Behavioral dif culties included frequent outbursts, irritability, and tantrums. There were no limitations in gross or fine motor func- Patient description tioning. At 4.5 years, he developed seizures characterized by eye deviation and automatisms. -

Supplementary Material
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Neurol Neurosurg Psychiatry Page 1 / 45 SUPPLEMENTARY MATERIAL Appendix A1: Neuropsychological protocol. Appendix A2: Description of the four cases at the transitional stage. Table A1: Clinical status and center proportion in each batch. Table A2: Complete output from EdgeR. Table A3: List of the putative target genes. Table A4: Complete output from DIANA-miRPath v.3. Table A5: Comparison of studies investigating miRNAs from brain samples. Figure A1: Stratified nested cross-validation. Figure A2: Expression heatmap of miRNA signature. Figure A3: Bootstrapped ROC AUC scores. Figure A4: ROC AUC scores with 100 different fold splits. Figure A5: Presymptomatic subjects probability scores. Figure A6: Heatmap of the level of enrichment in KEGG pathways. Kmetzsch V, et al. J Neurol Neurosurg Psychiatry 2021; 92:485–493. doi: 10.1136/jnnp-2020-324647 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Neurol Neurosurg Psychiatry Appendix A1. Neuropsychological protocol The PREV-DEMALS cognitive evaluation included standardized neuropsychological tests to investigate all cognitive domains, and in particular frontal lobe functions. The scores were provided previously (Bertrand et al., 2018). Briefly, global cognitive efficiency was evaluated by means of Mini-Mental State Examination (MMSE) and Mattis Dementia Rating Scale (MDRS). Frontal executive functions were assessed with Frontal Assessment Battery (FAB), forward and backward digit spans, Trail Making Test part A and B (TMT-A and TMT-B), Wisconsin Card Sorting Test (WCST), and Symbol-Digit Modalities test. -

Identification of ACSL4 As a Biomarker and Contributor of Ferroptosis In
Identication of ACSL4 as a biomarker and contributor of ferroptosis in clear cell renal cell carcinoma na guo ( [email protected] ) Primary research Keywords: Acyl-CoA synthetase long-chain family member 4 (ACSL4), biomarker, ferroptosis, clear cell renal cell carcinoma (ccRCC) Posted Date: May 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-24079/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/17 Abstract Background ACSL4 has been reported to be related to tumor genesis and involved in the processes of ferroptosis. However, the expression levels and prognostic value of ACSL4 in clear cell renal cell carcinoma (ccRCC) remain unclear. Methods The Oncomine and TCGA databases were used to predict the expression of ACSL4 mRNA in ccRCC and its association with ccRCC prognosis. The expression levels of ACSL4 were determined in human RCC tissues by real-time PCR. Kaplan-Meier curves were used to analyze the diagnostic and prognostic signicance of ACSL4 in ccRCC. A ferroptosis inducer (erastin) was used to investigate the effects of ACSL4 on ferroptosis in ccRCC cell lines. Results The expression level of ACSL4 was signicantly down-regulated in ccRCC tissues (P < 0.001), which was consistent with the analysis of the Oncomine and TCGA database. Then, immunohistochemical results demonstrated that the ACSL4 was weak or not detected in ccRCC tissues than that in normal tissues. ACSL4 differential expression level was signicantly related to gender, ccRCC subtypes, nodal invasion, tumor grade and cancer stages (all P < 0.001). Survival analysis revealed that overall survival was favorable in ccRCC patients with ACSL4 high expression (P = 0.014). -

Global Ubiquitylation Analysis of Mitochondria in Primary Neurons
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.01.438131; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Global ubiquitylation analysis of mitochondria in primary neurons identifies physiological Parkin targets following activation of PINK1 Odetta Antico1#, Alban Ordureau2#, Michael Stevens1, Francois Singh1, Marek φ Gierlinski3, Erica Barini1 , Mollie L. Rickwood1, Alan Prescott4, Rachel Toth1, Ian G. Ganley1, J. Wade Harper2*, and Miratul M. K. Muqit1* 1MRC Protein Phosphorylation and Ubiquitylation Unit, School of Life Sciences, University of Dundee, Dundee, United Kingdom, DD1 5EH, U.K. 2Department of Cell Biology, Harvard Medical School, Boston, MA 02115, USA 3Data Analysis Group, Division of Computational Biology, School of Life Sciences, University of Dundee, Dundee, United Kingdom, DD1 5EH, U.K. 4Dundee Imaging Facility, School of Life Sciences, University of Dundee, Dundee, United Kingdom, DD1 5EH, U.K #Denotes Equal Contribution φCurrent address: AbbVie Deutschland GmbH & Co, Knollstr, 67061, Ludwigshafen, Germany *Correspondence: [email protected] or [email protected] Keywords Neurons, PINK1, Parkin, ubiquitin, Parkinson’s disease, Mitochondria, Running Title: Ubiquitin analysis of mitochondria in neurons 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.04.01.438131; this version posted April 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -
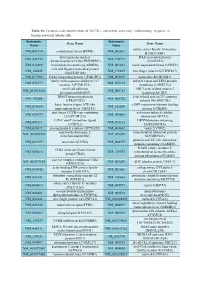
Systematic Name Gene Name Systematic Name Gene Name NM 001710 Complement Factor B(CFB) NM 052831 Solute Carrier Family 18 Member
Table S1: Genome-wide identification of SGLT2i`s interaction with early inflammatory response in human proximal tubular cells. Systematic Systematic Gene Name Gene Name Name Name solute carrier family 18 member NM_001710 complement factor B(CFB) NM_052831 B1(SLC18B1) heterogeneous nuclear DAZ associated protein NM_031372 NM_170711 ribonucleoprotein D like(HNRNPDL) 1(DAZAP1) NM_014299 bromodomain containing 4(BRD4) NM_001261 cyclin dependent kinase 9(CDK9) cilia and flagella associated protein NM_182628 NM_178835 zinc finger protein 827(ZNF827) 100(CFAP100) NM_017906 PAK1 interacting protein 1(PAK1IP1) NM_024015 homeobox B4(HOXB4) family with sequence similarity 167 ankyrin repeat and LEM domain NM_053279 NM_015114 member A(FAM167A) containing 2(ANKLE2) small cell adhesion ARP3 actin related protein 3 NM_001031628 NM_005721 glycoprotein(SMAGP) homolog(ACTR3) TRAF3 interacting protein actin related protein 2/3 complex NM_147686 NM_005720 2(TRAF3IP2) subunit 1B(ARPC1B) basic leucine zipper ATF-like cAMP responsive element binding NM_018664 NM_182898 transcription factor 3(BATF3) protein 5(CREB5) zinc finger CCCH-type containing activation induced cytidine NM_025079 NM_020661 12A(ZC3H12A) deaminase(AICDA) C-X-C motif chemokine ligand DENN domain containing NM_001511 NM_015213 1(CXCL1) 5A(DENND5A) NM_025072 prostaglandin E synthase 2(PTGES2) NM_004665 vanin 2(VNN2) superoxide dismutase 2, mitochondrial ribosomal protein NM_001024465 NM_016070 mitochondrial(SOD2) S23(MRPS23) jumonji and AT-rich interaction NM_033199 urocortin 2(UCN2) NM_004973 -

Links Between Iron and Lipids: Implications in Some Major Human Diseases
pharmaceuticals Review Links Between Iron and Lipids: Implications in Some Major Human Diseases Stephanie Rockfield, Ravneet Chhabra, Michelle Robertson, Nabila Rehman, Richa Bisht and Meera Nanjundan * Department of Cell Biology, Microbiology and Molecular Biology, University of South Florida, Tampa, FL 336200, USA; srockfi[email protected] (S.R.); [email protected] (R.C.); [email protected] (M.R.); [email protected] (N.R.); [email protected] (R.B.) * Correspondence: [email protected]; Tel.: +1-813-974-8133 Received: 31 August 2018; Accepted: 19 October 2018; Published: 22 October 2018 Abstract: Maintenance of iron homeostasis is critical to cellular health as both its excess and insufficiency are detrimental. Likewise, lipids, which are essential components of cellular membranes and signaling mediators, must also be tightly regulated to hinder disease progression. Recent research, using a myriad of model organisms, as well as data from clinical studies, has revealed links between these two metabolic pathways, but the mechanisms behind these interactions and the role these have in the progression of human diseases remains unclear. In this review, we summarize literature describing cross-talk between iron and lipid pathways, including alterations in cholesterol, sphingolipid, and lipid droplet metabolism in response to changes in iron levels. We discuss human diseases correlating with both iron and lipid alterations, including neurodegenerative disorders, and the available evidence regarding the potential mechanisms underlying how iron may promote disease pathogenesis. Finally, we review research regarding iron reduction techniques and their therapeutic potential in treating patients with these debilitating conditions. We propose that iron-mediated alterations in lipid metabolic pathways are involved in the progression of these diseases, but further research is direly needed to elucidate the mechanisms involved. -

A Genome Wide Association Study of Fast Beta EEG in Families of European Ancestry
CORE Metadata, citation and similar papers at core.ac.uk Provided by IUPUIScholarWorks HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Int J Psychophysiol Manuscript Author . Author Manuscript Author manuscript; available in PMC 2018 May 01. Published in final edited form as: Int J Psychophysiol. 2017 May ; 115: 74–85. doi:10.1016/j.ijpsycho.2016.12.008. A Genome Wide Association Study of Fast Beta EEG in Families of European Ancestry Jacquelyn L. Meyers1,*, Jian Zhang1, Niklas Manz1,2, Madhavi Rangaswamy3, Chella Kamarajan1, Leah Wetherill4, David B. Chorlian1, Sun J. Kang5, Lance Bauer6, Victor Hesselbrock6, John Kramer7, Samuel Kuperman7, John I. Nurnberger Jr4, Jay Tischfield8, Jen Chyong Wang9, Howard J. Edenberg4,10, Alison Goate9, Tatiana Foroud4, and Bernice Porjesz1 1Henri Begleiter Neurodynamics Laboratory, Department of Psychiatry and Behavioral Sciences, SUNY Downstate Medical Center, Brooklyn, NY, USA 2Department of Physics, College of Wooster, Wooster, OH, USA 3Department of Psychology, Christ University, Bangalore, India 4Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN, USA 5Albany Stratton VA Medical Center, Albany, NY, USA 6University of Connecticut School of Medicine, Farmington, CT, USA 7Department of Psychiatry, University of Iowa, Iowa City, IA, USA 8Rutgers University, Piscataway, NJ, USA 9Icahn School of Medicine at Mt. Sinai, New York, NY, USA 10Department of Biochemistry and Molecular Biology, Indiana University School of Medicine, Indianapolis, IN, USA Abstract BACKGROUND—Differences in fast beta (20–28 Hz) electroencephalogram (EEG) oscillatory activity distinguish some individuals with psychiatric and substance use disorders, suggesting that it may be a useful endophenotype for studying the genetics of disorders characterized by neural hyper-excitability. -

Downregulation of Carnitine Acyl-Carnitine Translocase by Mirnas
Page 1 of 288 Diabetes 1 Downregulation of Carnitine acyl-carnitine translocase by miRNAs 132 and 212 amplifies glucose-stimulated insulin secretion Mufaddal S. Soni1, Mary E. Rabaglia1, Sushant Bhatnagar1, Jin Shang2, Olga Ilkayeva3, Randall Mynatt4, Yun-Ping Zhou2, Eric E. Schadt6, Nancy A.Thornberry2, Deborah M. Muoio5, Mark P. Keller1 and Alan D. Attie1 From the 1Department of Biochemistry, University of Wisconsin, Madison, Wisconsin; 2Department of Metabolic Disorders-Diabetes, Merck Research Laboratories, Rahway, New Jersey; 3Sarah W. Stedman Nutrition and Metabolism Center, Duke Institute of Molecular Physiology, 5Departments of Medicine and Pharmacology and Cancer Biology, Durham, North Carolina. 4Pennington Biomedical Research Center, Louisiana State University system, Baton Rouge, Louisiana; 6Institute for Genomics and Multiscale Biology, Mount Sinai School of Medicine, New York, New York. Corresponding author Alan D. Attie, 543A Biochemistry Addition, 433 Babcock Drive, Department of Biochemistry, University of Wisconsin-Madison, Madison, Wisconsin, (608) 262-1372 (Ph), (608) 263-9608 (fax), [email protected]. Running Title: Fatty acyl-carnitines enhance insulin secretion Abstract word count: 163 Main text Word count: 3960 Number of tables: 0 Number of figures: 5 Diabetes Publish Ahead of Print, published online June 26, 2014 Diabetes Page 2 of 288 2 ABSTRACT We previously demonstrated that micro-RNAs 132 and 212 are differentially upregulated in response to obesity in two mouse strains that differ in their susceptibility to obesity-induced diabetes. Here we show the overexpression of micro-RNAs 132 and 212 enhances insulin secretion (IS) in response to glucose and other secretagogues including non-fuel stimuli. We determined that carnitine acyl-carnitine translocase (CACT, Slc25a20) is a direct target of these miRNAs. -

Generation of Acsl4 Gene Knockout Mouse Model by CRISPR/Cas9-Mediated Genome Engineering
Research Article ISSN: 2574 -1241 DOI: 10.26717.BJSTR.2019.14.002506 Generation of Acsl4 Gene Knockout Mouse Model by CRISPR/Cas9-Mediated Genome Engineering Hongyan Ren*, Zaidong Hua, Yanzhen Bi and Xinmin Zheng Hubei Key Laboratory of Animal Embryo Engineering and Molecular Breeding, Hubei Academy of Agricultural Sciences, China *Corresponding author: Hongyan Ren, Key Laboratory of Animal Embryo & Molecular Breeding of Hubei Province, Hubei Academy of Agricultural Science, China ARTICLE INFO abstract January 18, 2019 Received: Acyl-CoA synthetase 4 (Acsl4) is involved in lipid synthesis and fatty acid degradation, Published: February 06, 2019 and disruption of its function may causes lipid metabolism disorder in various species. Herein we reported to generate Acsl4 knockout (KO) mice using CRISPR/Cas9 gene editing system. In this report, a large deletion of 12kb in the Acsl4 gene was generated by co- Citation: Hongyan R, Zaidong H, injection of Cas9 mRNA and two guide RNAs (sgRNAs) into mouse fertilized oocytes. Yanzhen B, Xinmin Z. Generation of Three mutant mice carried target mutations were examined by PCR analysis and direct Acsl4 Gene Knockout Mouse Model by CRISPR/Cas9-Mediated Genome mutant mouse used as the parental mating with wild mouse to produce the F1 generation. Engineering. Biomed J Sci & Tech Res Takensequencing. together, The our gene data modified demonstrate mice theremain large healthy fragment and deletion display of normal mouse behavior.Acsl4 gene One by 14(1)-2019. BJSTR. MS.ID.002506. Acsl4 gene; CRISPR/cas9 Keywords: CRISPR/cas9Abbreivations: for intramuscular the first time. fat (IMF); Acyl-CoA synthetase long chain family 4 (Acsl4); system; gene knockout; mouse Clustered regularly interspaced short palindromic repeats/CRISPR-associated systems (CRISPR-Cas); knockout (KO); protospacer adjacent motif (PAM); nuclear localization signals (NLS) Introduction for investigating its effects on intramuscular fat deposition. -

Perkinelmer Genomics to Request the Saliva Swab Collection Kit for Patients That Cannot Provide a Blood Sample As Whole Blood Is the Preferred Sample
Autism and Intellectual Disability TRIO Panel Test Code TR002 Test Summary This test analyzes 2429 genes that have been associated with Autism and Intellectual Disability and/or disorders associated with Autism and Intellectual Disability with the analysis being performed as a TRIO Turn-Around-Time (TAT)* 3 - 5 weeks Acceptable Sample Types Whole Blood (EDTA) (Preferred sample type) DNA, Isolated Dried Blood Spots Saliva Acceptable Billing Types Self (patient) Payment Institutional Billing Commercial Insurance Indications for Testing Comprehensive test for patients with intellectual disability or global developmental delays (Moeschler et al 2014 PMID: 25157020). Comprehensive test for individuals with multiple congenital anomalies (Miller et al. 2010 PMID 20466091). Patients with autism/autism spectrum disorders (ASDs). Suspected autosomal recessive condition due to close familial relations Previously negative karyotyping and/or chromosomal microarray results. Test Description This panel analyzes 2429 genes that have been associated with Autism and ID and/or disorders associated with Autism and ID. Both sequencing and deletion/duplication (CNV) analysis will be performed on the coding regions of all genes included (unless otherwise marked). All analysis is performed utilizing Next Generation Sequencing (NGS) technology. CNV analysis is designed to detect the majority of deletions and duplications of three exons or greater in size. Smaller CNV events may also be detected and reported, but additional follow-up testing is recommended if a smaller CNV is suspected. All variants are classified according to ACMG guidelines. Condition Description Autism Spectrum Disorder (ASD) refers to a group of developmental disabilities that are typically associated with challenges of varying severity in the areas of social interaction, communication, and repetitive/restricted behaviors.