Composition for Titanium Nitride Hard Mask and Etch Residue Removal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2005/0124724 A1 Burton Et Al
US 2005O124724A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0124724 A1 Burton et al. (43) Pub. Date: Jun. 9, 2005 (54) POLYMER COMPOSITIONS WITH (21) Appl. No.: 10/728,439 BIOACTIVE AGENT, MEDICAL ARTICLES, AND METHODS (22) Filed: Dec. 5, 2003 (75) Inventors: Scott A. Burton, Woodbury, MN (US); Publication Classification Patrick D. Hyde, Burnsville, MN (US); Prabhakara S. Rao, Maplewood, MN (51) Int. Cl." ....................................................... C08K 3/00 (US); Caroline M. Ylitalo, Stillwater, (52) U.S. Cl. .............................................................. 523/122 MN (US) (57) ABSTRACT Correspondence Address: A polymer composition that includes a hydrophilic polymer, 3M INNOVATIVE PROPERTIES COMPANY an optional Secondary organic polymer, and a bioactive PO BOX 33427 agent distributed therein, wherein the bioactive agent is ST. PAUL, MN 55133-3427 (US) Selected from the group consisting of a Silver compound, a copper compound, a Zinc compound, and combinations (73) Assignee: 3M Innovative Properties Company thereof. US 2005/O124724 A1 Jun. 9, 2005 POLYMER COMPOSITIONS WITH BOACTIVE comprising absorbent hydrophilic microparticles, a metal AGENT, MEDICAL ARTICLES, AND METHODS compound Selected from the group consisting of a Silver compound, a copper compound, a Zinc compound, and BACKGROUND combinations thereof, wherein the Silver compound has a 0001 Polymer compositions that include bioactive Solubility of at least 0.1 gram per liter in water; and a agents (e.g., antimicrobial agents) are used for a variety of hydroxide Source that converts the metal compound to the applications, particularly medical applications Such as corresponding metal oxide. The components are combined wound dressings and wound packing materials. Conven in a manner to incorporate the metal oxide within the tional antimicrobial agents include ionizable Silver com microparticles. -

WO 2017/104134 Al 22 June 2017 (22.06.2017) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/104134 Al 22 June 2017 (22.06.2017) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C09J 103/00 (2006.01) C07H 3/02 (2006.01) kind of national protection available): AE, AG, AL, AM, B27N3/00 (2006.01) C07H 3/04 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/JP20 16/005 13 1 HN, HR, HU, ID, IL, IN, IR, IS, KE, KG, KH, KN, KP, (22) International Filing Date: KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, 14 December 2016 (14. 12.2016) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, (26) Publication Language: English TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 2015-247280 18 December 201 5 (18. 12.2015) JP (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: HENKEL AG & CO. KGAA [DE/DE]; Hen- GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, kelstrasse 67, 40589 Duesseldorf (DE). -

Nextal Pegs Suite MSDS
SAFETY DATA SHEET NeXtal PEGs Suite Version 2.0 Revision Date 04/02/2020 Print Date 04/02/2020 SECTION 1. IDENTIFICATION Product name : NeXtal PEGs Suite Manufacturer or supplier's details Company : NeXtal 6201 Trust Dr Holland, OH 43528 USA Telephone : 419-740-6600 E-mail address : www.calibrescientific.com Emergency telephone : CHEMTREC USA & Canada 1-800-424-9300 Outside USA & Canada (703) 527-3887 Chemtrec ID# 696910 Recommended use of the chemical and restrictions on use Recommended use : Laboratory chemicals SECTION 2. HAZARDS IDENTIFICATION GHS Classification Acute toxicity (Oral) : Category 4 Skin irritation : Category 2 Eye irritation : Category 2A Carcinogenicity : Category 1B Specific target organ : Category 3 (Respiratory system) systemic toxicity - single exposure Acute aquatic toxicity : Category 2 Chronic aquatic toxicity : Category 2 GHS Label element 1 / 17 SAFETY DATA SHEET NeXtal PEGs Suite Version 2.0 Revision Date 04/02/2020 Print Date 04/02/2020 Hazard pictograms : Signal Word : Danger Hazard Statements : H302 Harmful if swallowed. H315 Causes skin irritation. H319 Causes serious eye irritation. H335 May cause respiratory irritation. H350 May cause cancer. H411 Toxic to aquatic life with long lasting effects. Precautionary Statements : Prevention: P201 Obtain special instructions before use. P280 Wear protective gloves/protective clothing/eye protection/ face protection. Response: P308 + P313 IF exposed or concerned: Get medical advice/ attention. Other hazards None known. SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTS -

Spectra Library Index Ichem/SDBS Raman Library
Ichem / SDBS Raman スタンダードライブラリー 株式会社 エス・ティ・ジャパン S p e c t r a L i b r a r y I n d e x (Ver. 40) Ichem / SDBS Raman Library ライブラリー名:ラマンスタンダードライブラリー 商品番号:60001-40 株式会社 エス・ティ・ジャパン 〒103-0014 東京都中央区日本橋蛎殻町 1-14-10 Tel: 03-3666-2561 Fax:03-3666-2658 http://www.stjapan.co.jp 1 【販売代理店】(株)テクノサイエンス Tel:043-206-0155 Fax:043-206-0188 https://www.techno-lab-co.jp/ Ichem / SDBS Raman スタンダードライブラリー 株式会社 エス・ティ・ジャパン ((1,2-DIETHYLETHYLENE)BIS(P-PHENYLENE))DIACETATE ((2-(3-BENZYLSULFONYL-4-METHYLCYCLOHEXYL)PROPYL)SULFONYLMETHYL)BENZENE ((2,4,6-TRIOXOHEXAHYDRO-5-PYRIMIDINYL)IMINO)DIACETIC ACID ((2-CARBOXYETHYL)IMINO)DIACETIC ACID ((2-HYDROXYETHYL)IMINO)DIACETIC ACID ((2-NITROBENZYL)IMINO)DIACETIC ACID ((2-SULFOETHYL)IMINO)DIACETIC ACID ((3-(1-BROMO-1-METHYLETHYL)-7-OXO-1,3,5-CYCLOHEPTATRIEN-1-YL)OXY)DIFLUOROBORANE ((N-BENZYLOXYCARBONYL-L-ISOLEUCYL)-L-PROLYL-L-PHENYLALANYL)-N(OMEGA)-NITRO-L-ARGININE 4-NITROBENZYL ESTER (-)-2-AMINO-1-BUTANOL (-)-2-AMINO-6-MERCAPTOPURINE RIBOSIDE (-)-6,8-P-MENTHADIEN-2-OL (-)-DIACETYL-L-TARTARIC ACID (-)-DIBENZOYL-L-TARTARIC ACID (-)-DI-P-ANISOYL-L-TARTARIC ACID (-)-DI-P-TOLUOYL-L-TARTARIC ACID (-)-KAURENE (-)-MENTHOL (-)-MYRTENOL (-)-N,N,N',N'-TETRAMETHYL-D-TARTARDIAMIDE (-)-N,N'-DIBENZYL-D-TARTRAMIDE (+)-1,3,3-TRIMETHYLNORBORNANE-2-ONE (+)-2-(2,4,5,7-TETRANITRO-9-FLUORENYLIDENEAMINOOXY)PROPIONIC ACID (+)-2-AMINO-1-BUTANOL (+)-2-PINENE (+)-3,9-DIBROMOCAMPHOR (+)-3-CARENE (+)-5-BROMO-2'-DEOXYURIDINE (+)-AMMONIUM 3-BROMO-8-CAMPHORSULFONATE (+)-CAMPHOR (+)-CAMPHOR OXIME (+)-CAMPHORIC ACID (+)-CATECHIN (+)-DI-P-ANISOYL-D-TARTARIC -

Nextal Amso4 Suite MSDS
SAFETY DATA SHEET NeXtal AmSO4 Suite Version 2.0 Revision Date 03/31/2020 Print Date 03/31/2020 SECTION 1. IDENTIFICATION Product name : NeXtal AmSO4 Suite Manufacturer or supplier's details Company : NeXtal 6201 Trust Dr Holland, OH 43528 USA Telephone : 419-740-6600 E-mail address : www.calibrescientific.com Emergency telephone : CHEMTREC USA & Canada 1-800-424-9300 Recommended use of the chemical and restrictions on use Recommended use : Laboratory chemicals SECTION 2. HAZARDS IDENTIFICATION GHS Classification Acute toxicity (Oral) : Category 4 Acute toxicity (Inhalation) : Category 3 Skin irritation : Category 2 Eye irritation : Category 2A Germ cell mutagenicity : Category 1B Carcinogenicity : Category 1A Reproductive toxicity : Category 1B Specific target organ systemic toxicity : Category 3 (Respiratory system) - single exposure 1 / 20 SAFETY DATA SHEET NeXtal AmSO4 Suite Version 2.0 Revision Date 03/31/2020 Print Date 03/31/2020 Specific target organ Category 1 systemic toxicity - repeated exposure Acute aquatic toxicity : Category 1 Chronic aquatic toxicity : Category 1 GHS Label element Hazard pictograms : Signal Word : Danger Hazard Statements : H302 Harmful if swallowed. H315 Causes skin irritation. H319 Causes serious eye irritation. H331 Toxic if inhaled. H335 May cause respiratory irritation. H340 May cause genetic defects. H350 May cause cancer. H360 May damage fertility or the unborn child. H372 Causes damage to organs through prolonged or repeated exposure. H410 Very toxic to aquatic life with long lasting effects. Precautionary Statements : Prevention: P201 Obtain special instructions before use. P260 Do not breathe dust/fumes/gas/mist/vapors/spray. P280 Wear protective gloves/ protective clothing/ eye protection/ face protection. Response: P308 + P313 IF exposed or concerned: Get medical advice/ attention. -

Titanium Nitride Hard Mask and Etch Residue Removal
(19) TZZ¥Z ZZ_T (11) EP 3 089 200 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 02.11.2016 Bulletin 2016/44 H01L 21/3213 (2006.01) H01L 21/311 (2006.01) (21) Application number: 16167813.1 (22) Date of filing: 29.04.2016 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB •Liu,Wen Dar GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Chupei City, Hsinchu Hsien 302 (TW) PL PT RO RS SE SI SK SM TR • Lee, Yi-Chia Designated Extension States: Chupei City, 30267 (TW) BA ME • Casteel Jr., William Jack Designated Validation States: Fountain Hill, PA 18015 (US) MA MD • Chen, Tianniu Westford, MA 01886 (US) (30) Priority: 01.05.2015 US 201562155794 P • Agarwal, Rajiv Krishan 20.05.2015 US 201562164293 P Malvern, PA 19355 (US) 21.01.2016 US 201662281658 P • Rao, Madhukar Bhaskara 26.04.2016 US 201615138835 Carlsbad, CA 92008 (US) (71) Applicant: AIR PRODUCTS AND CHEMICALS, INC. (74) Representative: Andrae | Westendorp Allentown, PA 18195-1501 (US) Patentanwälte Partnerschaft Uhlandstraße 2 80336 München (DE) (54) TITANIUM NITRIDE HARD MASK AND ETCH RESIDUE REMOVAL (57) Composition, method and system for PVD TiN long chain organic amines or polyalkylamine to improve hard mask removal from 28/20nm pattern wafers have removal/etching selectivity of PVD TiN vs CVD TiN. The been disclosed. The composition uses peroxide as oxi- composition further comprises long chain organic acids dizing agent for PVD TiN hard mask removal under slight- or amines to maintain Co compatibility. -
TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia
United States Office Pollution EPA 745-R-00-005 Environmental Protection Prevention and Toxics Revised April 2018 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY Guidance for Reporting Aqueous Ammonia Section 313 of the Emergency Planning and Community Right-to-Know Act of 1986 (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. EPCRA section 313 is also known as the Toxics Release Inventory (TRI). CONTENTS Section 1. Introduction ............................................................................................................ 3 1.1 Who Must Report ...................................................................................... 3 1.2 Thresholds ................................................................................................. 3 1.3 Chemical Sources of Aqueous Ammonia ................................................. 4 1.4 De Minimis Concentrations ...................................................................... -
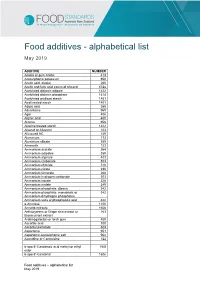
Food Additives - Alphabetical List
Food additives - alphabetical list May 2019 ADDITIVE NUMBER Acacia or gum Arabic 414 Acesulphame potassium 950 Acetic acid, glacial 260 Acetic and fatty acid esters of glycerol 472a Acetylated distarch adipate 1422 Acetylated distarch phosphate 1414 Acetylated oxidised starch 1451 Acid treated starch 1401 Adipic acid 355 Advantame 969 Agar 406 Alginic acid 400 Alitame 956 Alkaline treated starch 1402 Alkanet or Alkannin 103 Allura red AC 129 Aluminium 173 Aluminium silicate 559 Amaranth 123 Ammonium acetate 264 Ammonium adipates 359 Ammonium alginate 403 Ammonium carbonate 503 Ammonium chloride 510 Ammonium citrate 380 Ammonium fumarate 368 Ammonium hydrogen carbonate 503 Ammonium lactate 328 Ammonium malate 349 Ammonium phosphate, dibasic 342 Ammonium phosphate, monobasic or 342 Ammonium dihydrogen phosphates Ammonium salts of phosphatidic acid 442 α-Amylase 1100 Annatto extracts 160b Anthocyanins or Grape skin extract or 163 Blackcurrant extract Arabinogalactan or larch gum 409 Ascorbic acid 300 Ascorbyl palmitate 304 Aspartame 951 Aspartame-acesulphame salt 962 Azorubine or Carmoisine 122 b-apo-8′-Carotenoic acid methyl or ethyl 160f ester b-apo-8′-Carotenal 160e Food additives – alphabetical list May 2019 Beeswax, white and yellow 901 Beet red 162 Bentonite 558 Benzoic acid 210 Bleached starch 1403 Bone phosphate 542 Brilliant black BN or Brilliant Black PN 151 Brilliant Blue FCF 133 Brown HT 155 Butane 943a Butylated hydroxyanisole 320 Butylated hydroxytoluene 321 Calcium acetate 263 Calcium alginate 404 Calcium aluminium silicate -

Biological Stains, Sulphonpthalein Dyes & Bulk Chemicals
BIOLOGICAL STAINS & SULPHONPTHALEIN DYES Code Product Name CAS No. C.I. No. Code Product Name CAS No. C.I. No. ACID FUCHSIN 3244-88-0 42685 JANUS GREEN B 2869-83-2 11050 ALIZARIN RED S 130-22-3 -- LEISHMAN'S STAIN 12627-53-1 -- ALIZARIN COMPLEXONE MALACHITE GREEN 13425-25-7 42000 ALKALI BLUE 6B 1324-80-7 42750 METHYL ORANGE 547-58-0 13025 METHYL BLUE ACID 28983-56-4 42780 META CRESOL PURPLE BLUE 93 AURIN (Rosolic Acid) 603-45-2 43800 MAGNESON I -- -- -- AZURE 1 (B) 531-55-5 52010 May & Grunwald Stain 62851-42-7 -- AZURE A 531-53-3 52005 METHYLENE BLUE 7220-79-3 52015 AZUR II 37247-10-2 -- METHYL RED 493-52-7 13020 BIEBRICH SCARLET 4196-99-0 26905 METHYL GREEN 7114-03-6 BASIC FUCHSIN 632-99-5 42510 NEW FUCHSIN 3248-91-7 42520 BROMOPHENOL BLUE 115-39-9 NILE BLUE (SULFATE) 3625-57-8 51180 BROMOCRESOL GREEN 76-60-8 NITROSO R-SALT 525-05-3 BROMOCRESOL PURPLE 115-40-2 NEUTRAL RED 553-24-2 50040 BROMOCRESOL PURPLE 62625-28-9 ORANGE G 1936-15-8 16230 SODIUM SALT BROMOTHYMOL BLUE 76-59-5 O-CRESOLPHTHALEIN 59-27-0 BROMOTHYMOL BLUE 34722-95-93 PHENOL RED 143-74-8 SODIUM SALT PHENOL RED SODIUM CRESOL RED 1733-12-6 34487-61-1 SALT CHLOROPHENOL RED 4430-20-0 PHLOXINE B 18472-87-2 45410 CHROME AZUROL S 1667-99-8 43825 PAN INDICATOR 85-85-8 CRYSTAL VIOLET 548-62-9 42555 PAR (Mono Sodium) 16593-81-0 O-CRESOLPHTHALEIN 59-27-0 PONCEAU S 6226-79-5 27195 CALCON 2538-85-4 15705 P-ROSANILINE HCL 569-61-9 42500 CALCON CARBOXYLIC 3737-95-9 -- PHENOLPHTHALEIN ACID CALMAGITE 3147-14-6 -- ROSE BENGAL 632-69-9 45440 CONGO RED 573-58-0 22120 SAFRANIN 477-73-6 50240 -

Chemical Reagents & Solutions List
KASD 100ml 500ml 1 Litre 2.5 Litres 5 Litres Other Quantities ACETATE BUFFER 1.9M, pH 4.2 ACETATE BUFFER 4.1 M, pH 4.2 ACETATE BUFFER 2.4M, pH 4.5 ACETATE BUFFER 1M, pH 4.6 ACETATE BUFFER, pH 4.6 ACETATE BUFFER, pH 5.2 ACETIC ACID SOLUTION App-Chem MT1050/1 ACETIC ACID 0.010N (standardised) (.010M) ACETIC ACID 0.10N (standardised) (0.10M) ACETIC ACID 0.1N (unstandardised) ACETIC ACID 1% (unstandardised) ACETIC ACID 1.2% (unstandardised) ACETIC ACID 1M (unstandardised) ACETIC ACID 2M (unstandardised) ACETIC ACID 2.4% (unstandardised) ACETIC ACID 5M (unstandardised) ACETIC ACID 5% (unstandardised) ACETIC ACID 6% (unstandardised) ACETIC ACID 10% (unstandardised) ACETIC ACID 20% (unstandardised) ACETIC ACID 30% (unstandardised) ACETIC ACID 50% (unstandardised) ACETIC ACID Du Bois #1000 ACETIC ACID neutral to perchloric acid ACETOCARMINE ACETONE ALCOHOL ACETO-ORCEINE ACID ALCOHOL 3% ACID ALCOHOL 10% ACIDITY INDICATOR (MIXED INDICATOR for HCl) ACID MOLYBDATE (Phosphate) App-Chem RP5101 ACID MOLYBDATE (Silica) App-Chem RP5615 ACID SODIUM CHROMATE ACID SODIUM DICHROMATE ACID STARCH ACIDIFIER App-Chem RT1230A ACID STARCH INDICATOR App-Chem RT1230 ACID STARCH INDICATOR App-Chem RT1230p ACID STARCH powder Du Bois #725 ALBERT'S IODINE ALBERT’S STAIN ALCIAN BLUE 1% aqueous ALIQUAT 1% in DIBK ALIZAROL 68 milk test indicator ALIZAROL 72 milk test indicator ALIZAROL 90 milk test indicator ALKALI BLUE indicator (oil alkalinity) ALKALINE CUPROUS CHLORIDE ALKALINE PYROGALLOL ALKALINE SODIUM HYPOBROMITE ALKALINITY 1000 ppm ALKALINITY-M INDICATOR ALKALINITY-P -

EPA Emergency Planning and Community Right- To-Know Act Section 313 Reporting Guidance for the Leather Tanning and Finishing Industry TABLE of CONTENTS Page
United States Office of Pollution EPA 745-B-00-012 Environmental Protection Prevention and Toxics April 2000 Agency Washington, DC 20460 EPA Emergency Planning and Community Right- To-Know Act Section 313 Reporting Guidance for the Leather Tanning and Finishing Industry TABLE OF CONTENTS Page ACKNOWLEDGMENT ....................................................... vi OVERVIEW ............................................................... vii CHAPTER 1 - INTRODUCTION ............................................... 1-1 1.0 PURPOSE ..................................................... 1-1 1.1 Background on EPCRA Section 313 and PPA Section 6607 ........ 1-2 CHAPTER 2 - REPORTING REQUIREMENTS................................... 2-1 2.0 PURPOSE ....................................................... 2-1 2.1 Must You Report?......................................... 2-2 2.2 SIC Code Determination .................................... 2-4 2.3 Number of Employees...................................... 2-6 2.4 Manufacturing, Processing, and Otherwise Use of EPCRA Section 313 Chemicals or Chemical Categories ......................... 2-8 2.5 Activity Categories ....................................... 2-10 2.6 Persistent, Bioaccumulative, and Toxic (PBT) Chemicals ......... 2-13 2.7 How Do You Report? ..................................... 2-15 2.8 Form R................................................. 2-16 2.9 Alternate Threshold and Form A............................. 2-17 2.10 Trade Secrets ............................................ 2-18 2.11 Recordkeeping -

United States Patent (10) Patent No.: US 7.250.460 B1 Takamura (45) Date of Patent: *Jul
USOO7250.46OB1 (12) United States Patent (10) Patent No.: US 7.250.460 B1 Takamura (45) Date of Patent: *Jul. 31, 2007 (54) INORGANIC-ORGANIC POLYMER 3,951,676 A 4, 1976 Elste, Jr. NANOCOMPOSITE AND METHODS FOR 3,959,204 A 5/1976 Dunn ......................... 524/425 MAKING AND USING 4,251,416 A 2, 1981 Palmer ....................... 524/423 4,296,207 A 10/1981 Siegmund (75)75 Inventor: KoichiO O Takamura, Charlotte, NC (US) 4.462,8404,456,534. A * 7/19846/1984 SchillingLambert etet al.al. ............ 21Of 725 4,597,799 A 7/1986 Schilling (73) Assignee: BASF AG, Ludwigshafen 4,772,648 A 9/1988 Demangeon et al. Rheinland-Pfalz (DE) 4,921,892. A 5/1990 Moore et al. c - 0 4.944,804. A 7/1990 Schilling (*) Notice: Subject to any disclaimer, the term of this 5,160,453 A 1 1/1992 Schilling patent is extended or adjusted under 35 5,268,029 A 12/1993 Demangeon et al. U.S.C. 154(b) by 287 days. 5,443,632 A 8/1995 Schilling 5,667,718 A 9, 1997 Jones et al. This patent is Subject to a terminal dis- 5,843.222 A 12/1998 Miller et al. claimer. 6,000,876. A 12/1999 Shen et al. (21) Appl. No.: 10/828,457 FOREIGN PATENT DOCUMENTS GB 2151 640 7, 1985 (22) Filed: Apr. 20, 2004 RU 2O71491 * 1/1997 WO WO O2/O24759 3, 2002 Related U.S. Application Data WO WOO3,OOOT60 1, 2003 (63) Continuation-in-part of application No.