East Practice Management Guidelines Work Group: Update to Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of Antibiotics in Open Fractures of the Finger
Vol. 15A, No. 5 September 1990 Fasciectomy for treatment qf Dupuyren’s diseaw 16. Hueston JT. ‘Firebreak’ grafts in Dupuytren’s contrac- 20 Honner R, Lamb DW, James JIP. Dupuytren’s contrac- ture. Aust N Z J Surg 1984;54:277-81. ture. long term results after fasiectomy. J Bone Joint Surg 17. Strickland JW. The coverage of difficult digital defects 1971;53B:240-6. with local rotation flaps. In: Strickland JW, Steichen WB, 21 Green TL, Strickland JW, Torstrick RF. The proximal eds. Difficult problems in hand surgery. St. Louis: The interphalangeal joint in Dupuytren’s contracture. In: CV Mosby Company, 1982:38&l. Strickland JW, Steichen WB, eds. Difficult problems in 18. Hueston JT. Limited fasciectomy for Dupuytren’s con- hand surgery. St. Louis: The CV Mosby Company, tracture. Plast Reconstr Surg 1961;27:569-85. 1982:414-18. 19. Zachariae-L. Extensive versus limited fasciectomy for Dupuytren’s contracture. Stand J Plast Reconstr Surg 1967;1:150-3. Role of antibiotics in open fractures of the finger The role of antibiotics was investigated prospectively in 91 open fractures of the finger. Antibiotics were administered to alternate patients with open phalangeal fractures. Only finger fractures distal to the metacarpophalangeal joint were included. Both groups were treated with aggressive surgical irrigation and debridement. In four patients in each group clinical signs of infection eventually developed; osteomyelitis did not develop in any patients, and no secondary surgical procedures were required in either group. This data indicates that vigorous irrigation and debridement is adequate primary treatment for open phalangeal fractures in fingers with intact digital arteries. -

Open Supracondylar Humerus Fractures in Children
Open Access Original Article DOI: 10.7759/cureus.13903 Open Supracondylar Humerus Fractures in Children Tommy Pan 1 , Matthew R. Widner 1 , Michael M. Chau 2 , William L. Hennrikus 3 1. Department of Orthopaedics and Rehabilitation, Penn State College of Medicine, Hershey, USA 2. Department of Orthopaedic Surgery, University of Minnesota Twin Cities, Minneapolis, USA 3. Department of Orthopaedics and Rehabilitation, Penn State Health Milton S. Hershey Medical Center, Hershey, USA Corresponding author: Tommy Pan, [email protected] Abstract Purpose: Supracondylar humerus (SCH) fractures are the most common elbow fracture in children; however, they rarely occur as open injuries. Open fractures are associated with higher rates of infection, neurovascular injury, compartment syndrome, and nonunion. The purpose of this study was to evaluate the treatment and outcomes of open SCH fractures in children. Methods: Between 2008 and 2015, four children (1%) had open injuries among 420 treated for SCH fractures at a single center. The mean patient age was six years (range, four to eight years). Two patients had Gustilo- Anderson grade 1 open fractures and two had grade 2 fractures. Tetanus immunization was up-to-date in all. First dose of intravenous antibiotics was given on average 3hr 7min after onset of injury (range, 1hr 38min to 8hr 15min). Time from injury to irrigation and debridement (I&D) and closed reduction and percutaneous pinning (CRPP) was on average 8hr 16min (range, 4hr 19min to 13hr 15min). All patients received 24-hour intravenous antibiotics. Pins were removed at four weeks and bony union occurred by six weeks. Results: After an average follow-up period of 12 months (range, 6 to 22 months), there were no infections, neurovascular deficits, compartment syndromes, cubitus varus deformities, or range of motion losses. -

Upper Extremity Fractures
Department of Rehabilitation Services Physical Therapy Standard of Care: Distal Upper Extremity Fractures Case Type / Diagnosis: This standard applies to patients who have sustained upper extremity fractures that require stabilization either surgically or non-surgically. This includes, but is not limited to: Distal Humeral Fracture 812.4 Supracondylar Humeral Fracture 812.41 Elbow Fracture 813.83 Proximal Radius/Ulna Fracture 813.0 Radial Head Fractures 813.05 Olecranon Fracture 813.01 Radial/Ulnar shaft fractures 813.1 Distal Radius Fracture 813.42 Distal Ulna Fracture 813.82 Carpal Fracture 814.01 Metacarpal Fracture 815.0 Phalanx Fractures 816.0 Forearm/Wrist Fractures Radius fractures: • Radial head (may require a prosthesis) • Midshaft radius • Distal radius (most common) Residual deformities following radius fractures include: • Loss of radial tilt (Normal non fracture average is 22-23 degrees of radial tilt.) • Dorsal angulation (normal non fracture average palmar tilt 11-12 degrees.) • Radial shortening • Distal radioulnar (DRUJ) joint involvement • Intra-articular involvement with step-offs. Step-off of as little as 1-2 mm may increase the risk of post-traumatic arthritis. 1 Standard of Care: Distal Upper Extremity Fractures Copyright © 2007 The Brigham and Women's Hospital, Inc. Department of Rehabilitation Services. All rights reserved. Types of distal radius fracture include: • Colle’s (Dinner Fork Deformity) -- Mechanism: fall on an outstretched hand (FOOSH) with radial shortening, dorsal tilt of the distal fragment. The ulnar styloid may or may not be fractured. • Smith’s (Garden Spade Deformity) -- Mechanism: fall backward on a supinated, dorsiflexed wrist, the distal fragment displaces volarly. • Barton’s -- Mechanism: direct blow to the carpus or wrist. -

Distal Radius Fracture
Distal Radius Fracture Osteoporosis, a common condition where bones become brittle, increases the risk of a wrist fracture if you fall. How are distal radius fractures diagnosed? Your provider will take a detailed health history and perform a physical evaluation. X-rays will be taken to confirm a fracture and help determine a treatment plan. Sometimes an MRI or CT scan is needed to get better detail of the fracture or to look for associated What is a distal radius fracture? injuries to soft tissues such as ligaments or Distal radius fracture is the medical term for tendons. a “broken wrist.” To fracture a bone means it is broken. A distal radius fracture occurs What is the treatment for distal when a sudden force causes the radius bone, radius fracture? located on the thumb side of the wrist, to break. The wrist joint includes many bones Treatment depends on the severity of your and joints. The most commonly broken bone fracture. Many factors influence treatment in the wrist is the radius bone. – whether the fracture is displaced or non-displaced, stable or unstable. Other Fractures may be closed or open considerations include age, overall health, (compound). An open fracture means a bone hand dominance, work and leisure activities, fragment has broken through the skin. There prior injuries, arthritis, and any other injuries is a risk of infection with an open fracture. associated with the fracture. Your provider will help determine the best treatment plan What causes a distal radius for your specific injury. fracture? Signs and Symptoms The most common cause of distal radius fracture is a fall onto an outstretched hand, • Swelling and/or bruising at the wrist from either slipping or tripping. -

Open Fracture Management
Clinical Practice Management Guidelines Open Fracture Management I. Classification of Open Fractures (Gustilo Classification) Type I: Open fracture with a skin wound <1 cm in length and clean. Type II: Open fracture with a laceration >1 cm in length without extensive soft tissue damage, flaps, or avulsions. Type III: Open segmental fracture with >10 cm wound with extensive soft tissue injury or a traumatic amputation. Special categories in Type III include gunshot fractures and open fractures caused by farm injuries. IIIA Adequate soft tissue coverage. IIIB Significant soft tissue loss with exposed bone that requires soft tissue transfer to achieve coverage. IIIC Associated vascular injury that requires repair for limb preservation. II. Antibiotics for Open Fractures A. Statement of the Problem An open fracture, in which the fracture fragments communicate with the environment through a break in the skin, increases the risk of infection and soft tissue complications. It is accepted that the risk of infection and incidence of limb loss correlate with the Gustilo type. The prompt administration of appropriate antibiotics is believed to reduce these complications. B. Recommendations Systemic antibiotic coverage directed at gram-positive organisms should be initiated as soon as possible after injury, within one hour of arrival from the scene, prior to transfer from outlying hospital. Cefazolin 1-2 gm every 8 hours. Clindamycin 900 mg every 8 hours (Pcn allergy). Additional gram-negative coverage should be added for type III fractures. Gentamicin 6 mg/kg daily for patients with normal renal function. Obese patients require dosing based on Dosing Weight. Piperacillin/Tazabactam may be used in place of Cefazolin/Gentamicin in the face of rhabomyolyis and in impaired renal function. -

A Recipe for Gas Gangrene – a Case Report Dr
International Journal of Medicine Research ISSN: 2455-7404; Impact Factor: RJIF 5.42 www.medicinesjournal.com Volume 1; Issue 2; May 2016; Page No. 18-19 Open fracture, diabetes and neglection: A recipe for gas gangrene – A case report Dr. Ganesh Singh Dharmshaktu M.S. (Orthopaedics), Assistant Professor, Dept. of orthopaedics, Government Medical College, Haldwani, Uttarakhand, India. Abstract Gas gangrene is a sinister infection that often results in serious limb morbidity and dismemberment of the extremity is the sole option in severe cases. The prevention and early, meticulous debridement may help modify the course of disease and prevent the condition in some instances. The knowledge of factors associated with increased risk is important to mitigate the problem of inappropriate assessment of the infection. The early decision of amputation, if salvage seems unlikely, proves critical in saving life of the patient. The associated condition of diabetes potentiates the presence of infection and compounds the problem. The occurrence of gas gangrene in the setting of fractures is limited to few reports or small series and as the incidence of the condition is on decline, its potential to occur in high risk group should not be taken lightly. We, hereby, present a case of severe gas gangrene of leg following fractures of both bones with small open wound that was neglected and led to fulminant infection with an untoward outcome. Keywords: Gas Gangrene, Infection, Clostridia, Open Fracture, Diabetes, Complication, Amputation, Management 1. Introduction necrosis in the event of history of trauma led to a diagnosis of Gas gangrene is potentially devastating condition caused by probable gas gangrene. -

Open Tibial Fracture in a Non-Compliant Patient: a Case Report
Journal of Functional Morphology and Kinesiology Case Report Open Tibial Fracture in a Non-Compliant Patient: A Case Report Samuele Pizzolo, Gianluca Testa * ID , Giacomo Papotto, Giuseppe Mobilia, Giovanni Di Stefano, Giuseppe Sessa and Vito Pavone ID Department of General Surgery and Medical Surgical Specialties, Section of Orthopaedics and Traumatology, AOU Policlinico-Vittorio Emanuele, University of Catania, Via Plebiscito 628, 95100 Catania, Italy; [email protected] (S.P.); [email protected] (G.P.); [email protected] (G.M.); [email protected] (G.D.S.); [email protected] (G.S.); [email protected] (V.P.) * Correspondence: [email protected]; Tel.: +39-095-7435398; Fax: +39-095-350611 Received: 18 June 2018; Accepted: 10 August 2018; Published: 11 August 2018 Abstract: Open tibial fractures represent the most frequent fractures of long bones, comprising approximately 1.9% of all fractures. Although locked intramedullary nailing is the gold standard for treating closed and unstable tibia diaphyseal fractures, for most exposed fractures, an external fixator can first be used, followed by conversion through an intramedullary nail. The present report describes the case of a 17-year-old male who presented with a complex multi-segmented displaced tibia fracture, type 42-C3, with exposure of IIIB type according to the Gustilo–Anderson classification, and with an attached disrupted fracture of peroneal malleolus, type 44-B2. External fixation was the preferred treatment method. Before the definitive surgical treatment, the patient had a second accident that caused refracture and damage to the soft tissues and external fixation system. This prolonged the time estimated for the conversion from the external fixator to the intramedullary nail. -
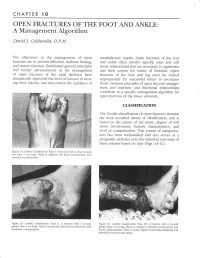
OPEN FRACTURES of the FOOT and ANKLE: a Management Algorithm
CHAPTER I O OPEN FRACTURES OF THE FOOT AND ANKLE: A Management Algorithm Dauid J. Caldarella, D.P.M. The objectives in the management of open unsatisfactory results. Open fractures of the foot fractures are to prer.ent infection, facilitate healing, and ankle often involve specific joint and soft and restore function. Established general principles tissue relationships that are necessary to appreciate and further advancements in the management and then restore for return of function. Open of open fractures of the axial skeleton have fractures of the foot and leg must be treated dramatically improved the level of success in treat- appropriately for successful return to pre-injury ing these injuries, and may reduce the incidence of levels. General principles of open fracture manage- ment and anatomic and functional relationships contribute to a specific management algorithm for open fractures of the lower extremity. CLq,SSIFICATION The Gustilo classification of open fractures remains the most accepted means of classification, and is based on the nature of the injury, degree of soft tissue involvement, fracture characteristics, and level of contamination. This system of categoriza- tion has been well-studied and also serves as a prognostic indicator as to the statistical outcomes of these injuries based on type (Figs. 1A-1C). Figure 1A. Gustilo Classiflcation Type L A ftacture with a clean wound less than 1 cm long. There is minimal soft tissr:e involvement, ancl minirnal contamination. % Figure 18, Gustilo Classification Type II. A fracture u,ith a w-ound Figure 1C. Gustilo Classification Type III. A fracture with a wound greater than 1 cm long. -

Results of Open Vs Closed Reduction and Internal Fixation of Type III
JK SCIENCE ORIGINALARTICLE Results of Open Vs Closed Reduction and Internal Fixation of Type III Supracondylar Fractures Anil Gupta, Mohinder Singh, Misbahul Haq Abstract The aim of the present study was to analyze the results of fixation of supracondylar fractures by open vs. closed reduction followed by internal fixation with k wires and assessing the union radiologically, complications associated with the procedure and restoration of range of motion and function of the elbow and to evaluate the results clinically regarding pain, stiffness, range of motion. A total of 40 cases were admitted for fracture supracondylar type 3. Out of them open reduction was done in 20 and in other 20 closed reduction was done. All 40 were fixed by internal fixation with k wires. The age of the patients in this study ranged from 4 yrs to 11 yrs. Males formed 75 %of the patients. 97.5 % fractures were extension types and the rest were flexion types. Left side was involved commonly (60 %). Duration from injury to surgery was an average of 23 hours. Mean procedure duration for closed group was 20 minutes and in open group was 70 minutes. Hospital stay in pt.s treated by closed reduction was 24 hrs (1 day). In patient treated by open reduction mean hospital stay was 5 days. Overall excellent results were found in 60 % in closed group and 35% in open group. Key Words Supracondylar Fractures, Gartland type 1 Fractuer, Introduction Material and Methods Supracondylar humerus fractures in children account This prospective study was conducted in the post for 60 % cases in elbow. -

Primary Vs. Secondary Closure of Open Fractures by Jenna Mackay, DPM1
The Northern Ohio Foot and Ankle Journal Official Publication of the NOFA Foundation Primary vs. Secondary Closure of Open Fractures by Jenna Mackay, DPM1 The Northern Ohio Foot and Ankle Journal 4(18):1-4 Abstract: Traditionally, the management of open fractures has included delayed or secondary closure. This method has been utilized to manage infection, and was first described by war-time physicians before the advent of modern antibiotics. While there is no one correct way to manage the soft tissues of an open fracture, primary closure should not be discounted as a viable treatment option. Based on a literature review, this article concludes that if proper debridement and antibiotic coverage can be obtained, primary closure of open fractures does not result in more instances of infection. Key words: Open fractures, primary closure, delayed primary closure, secondary closure, and Gustilo Anderson Accepted: December, 2017 Published: January, 2018 This is an Open Access article distributed under the terms of the Creative Commons Attribution License. It permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ©The Northern Ohio Foot and Ankle Foundation Journal. (www.nofafoundation.org) 2014. All rights reserved. INTRODUCTION commonly used open fracture classification system is The management of open fractures is an important the Gustilo Anderson classification (Figure 1). The topic with initial concepts originating from war-time Gustilo Anderson classification system not only surgeons1. Due to a lack of antibiotics in those times, organizes open fractures into groupings based on open fractures were most frequently left to heal by severity of open fracture but also provides secondary intention. -

Distal Humerus Fractures
Distal Humerus Fractures Edward J Harvey MD MSc FRCSC December 2015 Uploaded April 2016 Fractures of the Distal Humerus Previous authors and current contributors: Jeffrey J. Stephany, MD and Gregory J. Schmeling, MD; March 2004 Laura S. Phieffer, MD; Revised January 2006 Gregory J. Della Rocca, MD, PhD; Revised October 2010 Anatomy • Hinged joint with single axis of rotation (trochlear axis) – At bottom of virtual distal humeral triangle • Trochlea is center point of AP with a lateral and medial column 4-8 Deg. • Trochlear axis compared to longitudinal axis is 4-8 degrees in valgus Functional Anatomy • The distal humerus angles forward- like a hockey stick! • Lateral decubitus positioning during ORIF facilitates reconstruction • The trochlear axis is 3-8 degrees externally rotated 35-40 Deg. – (Least important to worry about if cartilage reconstructed) – Reason it is difficult to get a true lateral radiograph Evaluation • Physical exam – Soft tissue envelope – Vascular status • Radial and ulnar pulses – Neurologic status • Radial nerve - most commonly injured – 14 cm proximal to the lateral epicondyle – 20 cm proximal to the medial epicondyle • Median nerve - rarely injured • Ulnar nerve Fig. 33-7 Rockwood and Green Evaluation • Radiographic exam – Anterior-posterior and lateral radiographs – Traction views helpful • to evaluate intra-articular extension and for pre- operative planning (partial reduction via ligamentotaxis • Traction removes bone overlap – CT scan helpful in most cases • Comminuted capitellum or trochlea • Orientation -

Open Fractures of the Ankle: Management Options10.5005/Jp-Journals-10040-1074 and Factors Influencing Outcomes Symposium-Review Article
JFASJFAs (AP) Open Fractures of the Ankle: Management Options10.5005/jp-journals-10040-1074 and Factors influencing Outcomes SymPoSium-Review ARticle Open Fractures of the Ankle: Management Options and Factors influencing Outcomes 1Uttam C Saini, 2Mandeep S Dhillon, 3Udai Cheema, 4Rajesh K Rajnish ABSTRACT severe, often seen in young adult males, and frequently Open ankle fractures are rare injuries among all the ankle frac- neglected in polytrauma cases. Injuries of the bones con- tures and commonly occur after high-velocity trauma in road stituting the ankle (30.6%) are most common among the traffic accidents resulting in varying amounts of soft-tissue loss, foot injuries followed closely by metatarsal (27.9%) and periosteal stripping, microbial contamination, bone loss, and calcaneal fractures (21.4%).1,3 Open ankle fractures are vascular injury. Management of open ankle fractures remains a rare (3 to 6%) injuries among all the ankle fractures and daunting task due to the complex osseo-ligamentous complex, relatively thin soft-tissue coverage around the joint, propensity have also been increasingly seen in the elderly population 4 for wound infection and complications, and the risk of impaired besides the young trauma patients. These commonly functional ability. Management in the emergency trauma room occur after high-velocity trauma in road traffic accidents, includes initial stabilization of the patient, focused history, and resulting in varying amount of soft-tissue loss, periosteal detailed clinical evaluation determining the level and type of stripping, microbial contamination, bone loss, and vas- injury, extent of wound contamination, soft-tissue and/or bone cular injury. The classification of open ankle fractures loss, and neurovascular status of the injured limb followed by 5 radiographic evaluation.