Glucagon Receptor Signaling and Glucagon Resistance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corticotropin-Releasing Hormone Physiology
European Journal of Endocrinology (2006) 155 S71–S76 ISSN 0804-4643 Corticotropin-releasing hormone physiology Joseph A Majzoub Division of Endocrinology, Children’s Hospital Boston, Thomas Morgan Rotch Professor of Pediatrics, Harvard Medical School, 300 Longwood Avenue, Boston, Massachusetts 02115, USA (Correspondence should be addressed to J A Majzoub; Email: [email protected]) Abstract Corticotropin-releasing hormone (CRH), also known as corticotropin-releasing factor, is a highly conserved peptide hormone comprising 41 amino acid residues. Its name derives from its role in the anterior pituitary, where it mediates the release of corticotropin (ACTH) leading to the release of adrenocortical steroids. CRH is the major hypothalamic activator of the hypothalamic–pituitary– adrenal (HPA)axis. Major functions of the HPAinclude: (i) influencing fetal development of major organ systems including lung, liver, and gut, (ii) metabolic functions, including the maintenance of normal blood glucose levels during the fasting state via glycogenolysis and gluconeogenesis, (iii) modulation of immune function, and (iv) maintenance of cardiovascular tone. In addition, CRH, acting both directly and via the HPA, has a role in regulating several neuroendocrine functions including behavior, food intake, reproduction, growth, immune function, and autonomic function. CRH has been localized to the paraventricular nucleus (PVN) of the hypothalamus, which projects to the median eminence and other hypothalamic and midbrain targets. The CRH gene is composed of two exons. The CRH promoter contains a cAMP-response element, and the intron contains a restrictive element-1/neuron restrictive silencing element (RE-1/NRSE) sequence. Recently, a family of CRH-related peptides, termed the urocortins, has been identified. -

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -

Development of a System of High Ornithine and Citrulline Production by a Plant-Derived Lactic Acid Bacterium, Weissella Confusa K-28
Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28 㸦᳜≀⏤᮶ங㓟⳦ :HLVVHOODFRQIXVD. ࠾ࡅࡿ ࢜ࣝࢽࢳࣥཬࡧࢩࢺࣝࣜࣥ㧗⏕⏘ࢩࢫࢸ࣒ࡢᵓ⠏㸧 Md Rakhimuzzaman Student ID: D164180 Supervisor: Prof. Masanori Sugiyama Department of Probiotic Science for Preventive Medicine Graduate School of Biomedical and Health Sciences Hiroshima University, Japan 2019 ABBREVIATIONS ADI arginine deiminase AUC area under curve BLAST basic local alignment search tool CK carbamate kinase dNTP deoxyribonucleotide triphosphate DDBJ DNA Data Bank of Japan EDTA ethylenediamine tetra-acetic acid EtOH ethanol EPS exopolysaccharide GABA gamma-aminobutyric acid GRAS generally recognized as safe HPLC high performance liquid chromatography LAB lactic acid bacteria Lb. Lactobacillus OD optical density OTC ornithine carbamoyltransferase PITC phenyl isothiocyanate TAE tris-acetate-ethylenediamine tetra-acetic acid TE tris-ethylenediamine tetra-acetic acid TEA triethylamine Tris tris(hydroxymethyl)aminomethane rDNA ribosomal deoxyribonucleic acid RT-PCR reverse transcription polymerase chain reaction CONTENTS INTRODUCTION 1-17 OBJECTIVES 18 MATERIALS AND METHODS 19-34 RESULTS 35-57 DISCUSSIONS 58-65 CONCLUSION 66-67 ACKNOWLEDGEMENTS 68 REFERENCES 69-84 INTRODUCTION Lactic acid bacteria (LAB) are an order of gram-positive, acid-tolerant, generally nonsporulating, non-respiring, either rod-shaped or spherical microorganisms. Since LAB lack the ability of synthesizing porphyrins (heme, and components of respiratory chains) (Pessione et al, 2010), the bacteria can not generate ATP without external heme supplementation (Pessione et al, 2010). The LAB strains can only obtain ATP by fermentation, usually they generate the ATP from some sugars. Because LAB do not use oxygen for energy production, the bacteria easily grow under anaerobic conditions, but they can also grow in presence of oxygen. -
![LANTUS® (Insulin Glargine [Rdna Origin] Injection)](https://docslib.b-cdn.net/cover/0369/lantus%C2%AE-insulin-glargine-rdna-origin-injection-60369.webp)
LANTUS® (Insulin Glargine [Rdna Origin] Injection)
Rev. March 2007 Rx Only LANTUS® (insulin glargine [rDNA origin] injection) LANTUS® must NOT be diluted or mixed with any other insulin or solution. DESCRIPTION LANTUS® (insulin glargine [rDNA origin] injection) is a sterile solution of insulin glargine for use as an injection. Insulin glargine is a recombinant human insulin analog that is a long-acting (up to 24-hour duration of action), parenteral blood-glucose-lowering agent. (See CLINICAL PHARMACOLOGY). LANTUS is produced by recombinant DNA technology utilizing a non- pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, it is 21A- B B Gly-30 a-L-Arg-30 b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. It has the following structural formula: LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 IU (3.6378 mg) insulin glargine. Inactive ingredients for the 10 mL vial are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection. Inactive ingredients for the 3 mL cartridge are 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection. The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4. CLINICAL PHARMACOLOGY Mechanism of Action: The primary activity of insulin, including insulin glargine, is regulation of glucose metabolism. -

Metabolic Reprogramming in Prostate Cancer
www.nature.com/bjc REVIEW ARTICLE Metabolic reprogramming in prostate cancer Fahim Ahmad 1,2, Murali Krishna Cherukuri2 and Peter L. Choyke1 Although low risk localised prostate cancer has an excellent prognosis owing to effective treatments, such as surgery, radiation, cryosurgery and hormone therapy, metastatic prostate cancer remains incurable. Existing therapeutic regimens prolong life; however, they are beset by problems of resistance, resulting in poor outcomes. Treatment resistance arises primarily from tumour heterogeneity, altered genetic signatures and metabolic reprogramming, all of which enable the tumour to serially adapt to drugs during the course of treatment. In this review, we focus on alterations in the metabolism of prostate cancer, including genetic signatures and molecular pathways associated with metabolic reprogramming. Advances in our understanding of prostate cancer metabolism might help to explain many of the adaptive responses that are induced by therapy, which might, in turn, lead to the attainment of more durable therapeutic responses. British Journal of Cancer https://doi.org/10.1038/s41416-021-01435-5 BACKGROUND Several tools have been designed to assess metabolism without Cellular metabolism involves complex biochemical processes disturbing the system. Advances in nuclear magnetic resonance through which specific nutrients are consumed. Carbohydrates, and mass spectroscopy have helped to quantify the carbon influx fatty acids and amino acids are the main source of nutrients for of the central metabolic pathways (e.g. TCA cycle, glycolysis and energy homeostasis and macromolecular synthesis. They are the pentose phosphate pathway) in mammalian systems, and this main constituent of core metabolic pathways that can be approach has been instrumental in demonstrating that the classified as anabolic, catabolic or waste producing (Fig. -

Drug Information Center Highlights of FDA Activities
Drug Information Center Highlights of FDA Activities – 3/1/21 – 3/31/21 FDA Drug Safety Communications & Drug Information Updates: Ivermectin Should Not Be Used to Treat or Prevent COVID‐19: MedWatch Update 3/5/21 The FDA advised consumers against the use of ivermectin for the treatment or prevention of COVID‐19 following reports of patients requiring medical support and hospitalization after self‐medicating. Ivermectin has not been approved for this use and is not an anti‐viral drug. Health professionals are encouraged to report adverse events associated with ivermectin to MedWatch. COVID‐19 EUA FAERS Public Dashboard 3/15/21 The FDA launched an update to the FDA Adverse Event Reporting System (FAERS) Public Dashboard that provides weekly updates of adverse event reports submitted to FAERS for drugs and therapeutic biologics used under an Emergency Use Authorization (EUA) during the COVID‐19 public health emergency. Monoclonal Antibody Products for COVID‐19 – Fact Sheets Updated to Address Variants 3/18/21 The FDA authorized revised fact sheets for health care providers to include susceptibility of SARS‐CoV‐2 variants to each of the monoclonal antibody products available through EUA for the treatment of COVID‐19 (bamlanivimab, bamlanivimab and etesevimab, and casirivimab and imdevimab). Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm 3/25/21 The FDA warned that abuse and misuse of the nasal decongestant propylhexedrine, sold OTC in nasal decongestant inhalers, has been increasingly associated with cardiovascular and mental health problems. The FDA has recommended product design changes to support safe use, such as modifications to preclude tampering and limits on the content within the device. -

Expression of Urocortin and Corticotropin-Releasing Hormone Receptors in the Horse Thyroid Gland
Cell Tissue Res DOI 10.1007/s00441-012-1450-4 REGULAR ARTICLE Expression of urocortin and corticotropin-releasing hormone receptors in the horse thyroid gland Caterina Squillacioti & Adriana De Luca & Sabrina Alì & Salvatore Paino & Giovanna Liguori & Nicola Mirabella Received: 11 January 2012 /Accepted: 3 May 2012 # Springer-Verlag 2012 Abstract Urocortin (UCN) is a 40-amino-acid peptide and UCN, CRHR1 and CRHR2 and that UCN plays a role in a member of the corticotropin-releasing hormone (CRH) the regulation of thyroid physiological functions through a family, which includes CRH, urotensin I, sauvagine, paracrine mechanism. UCN2 and UCN3. The biological actions of CRH family peptides are mediated via two types of G-protein-coupled Keywords Follicular cells . C-cells . RT-PCR . receptors, namely CRH type 1 receptor (CRHR1) and CRH Immunohistochemistry . Horse type 2 receptor (CRHR2). The biological effects of these peptides are mediated and modulated not only by CRH receptors but also via a highly conserved CRH-binding Introduction protein (CRHBP). Our aim was to investigate the expression of UCN, CRHR1, CRHR2 and CRHBP by immunohisto- Urocortin (UCN) is a peptide of 40 amino acids and is a chemistry, Western blot and reverse transcription with the member of the corticotropin-releasing hormone (CRH) fam- polymerase chain reaction (RT-PCR) in the horse thyroid ily, which includes CRH, urotensin I, sauvagine, UCN2 and gland. The results showed that UCN, CRHR1 and CRHR2 UCN3. Vaughan et al. (1995) were the first to identify UCN, were expressed in the thyroid gland, whereas CRHBP was which exhibits 45% homology to CRH (Latchman 2002; not expressed. -

Searching for Novel Peptide Hormones in the Human Genome Olivier Mirabeau
Searching for novel peptide hormones in the human genome Olivier Mirabeau To cite this version: Olivier Mirabeau. Searching for novel peptide hormones in the human genome. Life Sciences [q-bio]. Université Montpellier II - Sciences et Techniques du Languedoc, 2008. English. tel-00340710 HAL Id: tel-00340710 https://tel.archives-ouvertes.fr/tel-00340710 Submitted on 21 Nov 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE MONTPELLIER II SCIENCES ET TECHNIQUES DU LANGUEDOC THESE pour obtenir le grade de DOCTEUR DE L'UNIVERSITE MONTPELLIER II Discipline : Biologie Informatique Ecole Doctorale : Sciences chimiques et biologiques pour la santé Formation doctorale : Biologie-Santé Recherche de nouvelles hormones peptidiques codées par le génome humain par Olivier Mirabeau présentée et soutenue publiquement le 30 janvier 2008 JURY M. Hubert Vaudry Rapporteur M. Jean-Philippe Vert Rapporteur Mme Nadia Rosenthal Examinatrice M. Jean Martinez Président M. Olivier Gascuel Directeur M. Cornelius Gross Examinateur Résumé Résumé Cette thèse porte sur la découverte de gènes humains non caractérisés codant pour des précurseurs à hormones peptidiques. Les hormones peptidiques (PH) ont un rôle important dans la plupart des processus physiologiques du corps humain. -
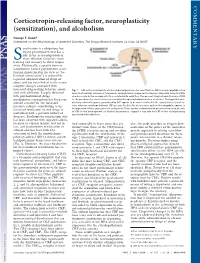
Corticotropin-Releasing Factor, Neuroplasticity (Sensitization), and Alcoholism
COMMENTARY Corticotropin-releasing factor, neuroplasticity (sensitization), and alcoholism George F. Koob* Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA 92037 ensitization is a ubiquitous bio- logical phenomenon that has a role in the neuroadaptation of many different functions, from Slearning and memory to stress respon- sivity. Historically, a specific form of sensitization termed psychomotor sensi- tization (mistermed in my view as ‘‘be- havioral sensitization’’) is induced by repeated administration of drugs of abuse and has been linked to the neuro- adaptive changes associated with increased drug-seeking behavior associ- Fig. 1. CNS actions relevant to alcohol-induced psychomotor sensitization. CRF is a neuropeptide in the ated with addiction. Largely observed brain that controls autonomic, hormonal, and behavioral responses to stressors. New data show that CRF with psychostimulant drugs, also has a role in the neuroplasticity associated with addiction. The present study extends the role of CRF psychomotor sensitization has been con- to the psychomotor sensitization associated with repeated administration of alcohol. The hypothalamic- sidered a model for the increased pituitary-adrenal responses produced by CRF appear to be more involved in the acquisition of sensitiza- incentive salience contributing to the tion, whereas extrahypothalamic CRF systems, likely to be in structures such as the amygdala, appear to increased motivation to seek drugs in be important for the expression of sensitization. These results, combined with previous studies on the role of CRF in the development of alcohol dependence, suggest a key role for CRF in the neuroplasticity individuals with a previous history of associated with addiction. -

Urocortin 2 Modulates Glucose Utilization and Insulin Sensitivity in Skeletal Muscle
Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle Alon Chen*†, Bhawanjit Brar*, Cheol Soo Choi‡, David Rousso*, Joan Vaughan*, Yael Kuperman†, Shee Ne Kim‡, Cindy Donaldson*, Sean M. Smith*, Pauline Jamieson*, Chien Li*, Tim R. Nagy§, Gerald I. Shulman‡, Kuo-Fen Lee*, and Wylie Vale*¶ *Clayton Foundation Laboratories for Peptide Biology, The Salk Institute for Biological Studies, La Jolla, CA 92037; †Department of Neurobiology, The Weizmann Institute of Science, Rehovot 76100, Israel; ‡Department of Internal Medicine and Cellular and Molecular Physiology and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06536; and §Department of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL 35294 Contributed by Wylie Vale, August 28, 2006 Skeletal muscle is the principal tissue responsible for insulin- Results and Discussion stimulated glucose disposal and is a major site of peripheral insulin Generation of Ucn 2-Deficient Mice. To explore the physiological resistance. Urocortin 2 (Ucn 2), a member of the corticotropin- role of Ucn 2, we generated mice that are deficient in this releasing factor (CRF) family, and its cognate type 2 CRF receptor peptide. A genomic DNA clone containing Ucn 2 was isolated, (CRFR2) are highly expressed in skeletal muscle. To determine the and a targeting construct in which the full Ucn 2 coding sequence physiological role of Ucn 2, we generated mice that are deficient in was replaced with a neomycin-resistant gene cassette was gen- this peptide. Using glucose-tolerance tests (GTTs), insulin-tolerance erated (Fig. 1a). J1 ES cells were electroporated with the tests (ITTs), and hyperinsulinemic euglycemic glucose clamp stud- targeting construct and were selected as described in ref. -

Amino Acid Degradation; Urea Cycle
Amino acid degradation; urea cycle Kristina Mlinac Jerković [email protected] In animals, amino acids undergo oxidative degradation in three different metabolic circumstances: 1. During the normal synthesis and degradation of cellular proteins, some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. 2. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. 3. During starvation or in uncontrolled diabetes mellitus, when carbohydrates are either unavailable or not properly utilized, cellular proteins are used as fuel. Metabolic fates of amino groups - The metabolism of nitrogen-containing molecules such as proteins and nucleic acids differs significantly from that of carbohydrates and lipids. - Whereas the latter molecules can be stored and mobilized as needed for biosynthetic reactions or for energy generation, there is no nitrogen-storing molecule (one exception to this rule is storage protein in seeds). - Organisms must constantly replenish their supply of usable nitrogen to replace organic nitrogen that is lost in catabolism. For example, animals must have a steady supply of amino acids in their diets to replace the nitrogen excreted as urea, uric acid, and other nitrogenous waste products. - Since excess dietary amino acids are neither stored for future use nor excreted, they are converted to common metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-keto-glutarate. - Consequently, amino acids are also precursors of glucose, fatty acids, and ketone bodies and are therefore metabolic fuels. -

Protein Metabolism and It's Disorders
Protein Metabolism and it’s Disorders (Course : M.Sc. Zoology) Course Code: ZOOL 4008 (Biochemistry and Metabolism), Semester –II Dr. Shyam Babu Prasad Assistant Professor Department of Zoology Mahatma Gandhi Central University (MGCU), Motihari-845401 (Bihar) Email: [email protected] Important facts of the Protein Metabolism •Proteins metabolism referred as synthesis of protein from Amino acids (Anabolism) and breakdown of proteins (Catabolism). •In the Unit II, we discussed the synthesis (Anabolism) of various form of the proteins from different amino acids. • Here we mainly focused on the Catabolism of the protein, which is integral part of metabolism of the nitrogen-containing molecules. • Nitrogen enters in to the body in various form of compounds that is present in food, the most important is amino acids (as dietary food). • During Protein/amino acid metabolism (Catabolism), Nitrogen leaves the body as urea, ammonia, and in form of other derivatives. • The Catabolism of the protein depends on the amino acid pool and protein turnover (the rate of protein synthesis and degradation is equal). • The amino acids present in through out of the body (cells, blood, and the extracellular fluids etc) is called amino acid pools. It is maintained by following factors as represented in below diagram: Body protein Dietary degradation Protein 400gm/day 600gm/day Supplied Amino acid pool Depleted Synthesis of Remaining Synthesis of Glucose, Synthesis of amino acids Nitrogen containing Glycogen, the Body burned as compound like Ketone Proteins