How Does Nitrogen Enter the Biomass, and Form Amino Acids Overview of the Flow of Nitrogen in the Biosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of a System of High Ornithine and Citrulline Production by a Plant-Derived Lactic Acid Bacterium, Weissella Confusa K-28
Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28 㸦᳜≀⏤᮶ங㓟⳦ :HLVVHOODFRQIXVD. ࠾ࡅࡿ ࢜ࣝࢽࢳࣥཬࡧࢩࢺࣝࣜࣥ㧗⏕⏘ࢩࢫࢸ࣒ࡢᵓ⠏㸧 Md Rakhimuzzaman Student ID: D164180 Supervisor: Prof. Masanori Sugiyama Department of Probiotic Science for Preventive Medicine Graduate School of Biomedical and Health Sciences Hiroshima University, Japan 2019 ABBREVIATIONS ADI arginine deiminase AUC area under curve BLAST basic local alignment search tool CK carbamate kinase dNTP deoxyribonucleotide triphosphate DDBJ DNA Data Bank of Japan EDTA ethylenediamine tetra-acetic acid EtOH ethanol EPS exopolysaccharide GABA gamma-aminobutyric acid GRAS generally recognized as safe HPLC high performance liquid chromatography LAB lactic acid bacteria Lb. Lactobacillus OD optical density OTC ornithine carbamoyltransferase PITC phenyl isothiocyanate TAE tris-acetate-ethylenediamine tetra-acetic acid TE tris-ethylenediamine tetra-acetic acid TEA triethylamine Tris tris(hydroxymethyl)aminomethane rDNA ribosomal deoxyribonucleic acid RT-PCR reverse transcription polymerase chain reaction CONTENTS INTRODUCTION 1-17 OBJECTIVES 18 MATERIALS AND METHODS 19-34 RESULTS 35-57 DISCUSSIONS 58-65 CONCLUSION 66-67 ACKNOWLEDGEMENTS 68 REFERENCES 69-84 INTRODUCTION Lactic acid bacteria (LAB) are an order of gram-positive, acid-tolerant, generally nonsporulating, non-respiring, either rod-shaped or spherical microorganisms. Since LAB lack the ability of synthesizing porphyrins (heme, and components of respiratory chains) (Pessione et al, 2010), the bacteria can not generate ATP without external heme supplementation (Pessione et al, 2010). The LAB strains can only obtain ATP by fermentation, usually they generate the ATP from some sugars. Because LAB do not use oxygen for energy production, the bacteria easily grow under anaerobic conditions, but they can also grow in presence of oxygen. -

Metabolic Reprogramming in Prostate Cancer
www.nature.com/bjc REVIEW ARTICLE Metabolic reprogramming in prostate cancer Fahim Ahmad 1,2, Murali Krishna Cherukuri2 and Peter L. Choyke1 Although low risk localised prostate cancer has an excellent prognosis owing to effective treatments, such as surgery, radiation, cryosurgery and hormone therapy, metastatic prostate cancer remains incurable. Existing therapeutic regimens prolong life; however, they are beset by problems of resistance, resulting in poor outcomes. Treatment resistance arises primarily from tumour heterogeneity, altered genetic signatures and metabolic reprogramming, all of which enable the tumour to serially adapt to drugs during the course of treatment. In this review, we focus on alterations in the metabolism of prostate cancer, including genetic signatures and molecular pathways associated with metabolic reprogramming. Advances in our understanding of prostate cancer metabolism might help to explain many of the adaptive responses that are induced by therapy, which might, in turn, lead to the attainment of more durable therapeutic responses. British Journal of Cancer https://doi.org/10.1038/s41416-021-01435-5 BACKGROUND Several tools have been designed to assess metabolism without Cellular metabolism involves complex biochemical processes disturbing the system. Advances in nuclear magnetic resonance through which specific nutrients are consumed. Carbohydrates, and mass spectroscopy have helped to quantify the carbon influx fatty acids and amino acids are the main source of nutrients for of the central metabolic pathways (e.g. TCA cycle, glycolysis and energy homeostasis and macromolecular synthesis. They are the pentose phosphate pathway) in mammalian systems, and this main constituent of core metabolic pathways that can be approach has been instrumental in demonstrating that the classified as anabolic, catabolic or waste producing (Fig. -

Lecture: 28 TRANSAMINATION, DEAMINATION and DECARBOXYLATION
Lecture: 28 TRANSAMINATION, DEAMINATION AND DECARBOXYLATION Protein metabolism is a key physiological process in all forms of life. Proteins are converted to amino acids and then catabolised. The complete hydrolysis of a polypeptide requires mixture of peptidases because individual peptidases do not cleave all peptide bonds. Both exopeptidases and endopeptidases are required for complete conversion of protein to amino acids. Amino acid metabolism The amino acids not only function as energy metabolites but also used as precursors of many physiologically important compounds such as heme, bioactive amines, small peptides, nucleotides and nucleotide coenzymes. In normal human beings about 90% of the energy requirement is met by oxidation of carbohydrates and fats. The remaining 10% comes from oxidation of the carbon skeleton of amino acids. Since the 20 common protein amino acids are distinctive in terms of their carbon skeletons, amino acids require unique degradative pathway. The degradation of the carbon skeletons of 20 amino acids converges to just seven metabolic intermediates namely. i. Pyruvate ii. Acetyl CoA iii. Acetoacetyl CoA iv. -Ketoglutarate v. Succinyl CoA vi. Fumarate vii. Oxaloacetate Pyruvate, -ketoglutarate, succinyl CoA, fumarate and oxaloacetate can serve as precursors for glucose synthesis through gluconeogenesis.Amino acids giving rise to these intermediates are termed as glucogenic. Those amino acids degraded to yield acetyl CoA or acetoacetate are termed ketogenic since these compounds are used to synthesize ketone bodies. Some amino acids are both glucogenic and ketogenic (For example, phenylalanine, tyrosine, tryptophan and threonine. Catabolism of amino acids The important reaction commonly employed in the breakdown of an amino acid is always the removal of its -amino group. -

Amino Acid Degradation; Urea Cycle
Amino acid degradation; urea cycle Kristina Mlinac Jerković [email protected] In animals, amino acids undergo oxidative degradation in three different metabolic circumstances: 1. During the normal synthesis and degradation of cellular proteins, some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. 2. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. 3. During starvation or in uncontrolled diabetes mellitus, when carbohydrates are either unavailable or not properly utilized, cellular proteins are used as fuel. Metabolic fates of amino groups - The metabolism of nitrogen-containing molecules such as proteins and nucleic acids differs significantly from that of carbohydrates and lipids. - Whereas the latter molecules can be stored and mobilized as needed for biosynthetic reactions or for energy generation, there is no nitrogen-storing molecule (one exception to this rule is storage protein in seeds). - Organisms must constantly replenish their supply of usable nitrogen to replace organic nitrogen that is lost in catabolism. For example, animals must have a steady supply of amino acids in their diets to replace the nitrogen excreted as urea, uric acid, and other nitrogenous waste products. - Since excess dietary amino acids are neither stored for future use nor excreted, they are converted to common metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-keto-glutarate. - Consequently, amino acids are also precursors of glucose, fatty acids, and ketone bodies and are therefore metabolic fuels. -

Protein Metabolism and It's Disorders
Protein Metabolism and it’s Disorders (Course : M.Sc. Zoology) Course Code: ZOOL 4008 (Biochemistry and Metabolism), Semester –II Dr. Shyam Babu Prasad Assistant Professor Department of Zoology Mahatma Gandhi Central University (MGCU), Motihari-845401 (Bihar) Email: [email protected] Important facts of the Protein Metabolism •Proteins metabolism referred as synthesis of protein from Amino acids (Anabolism) and breakdown of proteins (Catabolism). •In the Unit II, we discussed the synthesis (Anabolism) of various form of the proteins from different amino acids. • Here we mainly focused on the Catabolism of the protein, which is integral part of metabolism of the nitrogen-containing molecules. • Nitrogen enters in to the body in various form of compounds that is present in food, the most important is amino acids (as dietary food). • During Protein/amino acid metabolism (Catabolism), Nitrogen leaves the body as urea, ammonia, and in form of other derivatives. • The Catabolism of the protein depends on the amino acid pool and protein turnover (the rate of protein synthesis and degradation is equal). • The amino acids present in through out of the body (cells, blood, and the extracellular fluids etc) is called amino acid pools. It is maintained by following factors as represented in below diagram: Body protein Dietary degradation Protein 400gm/day 600gm/day Supplied Amino acid pool Depleted Synthesis of Remaining Synthesis of Glucose, Synthesis of amino acids Nitrogen containing Glycogen, the Body burned as compound like Ketone Proteins -
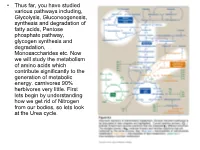
Transaminations and Urea Cycle
• Thus far, you have studied various pathways including, Glycolysis, Gluconeogenesis, synthesis and degradation of fatty acids, Pentose phosphate pathway, glycogen synthesis and degradation, Monosaccharides etc. Now we will study the metabolism of amino acids which contribute significantly to the generation of metabolic energy. carnivores 90% herbivores very little. First lets begin by understanding how we get rid of Nitrogen from our bodies, so lets look at the Urea cycle. 1 α Amino group keeps amino acids safe from oxidative breakdown. Removal of this group is essential for energy production it is an obligatory step for catabolism. Nitrogen can then be incorporated or excreted.This is done through transamination or oxidative deamination, provide amonia and aspartate sources for the Urea cycle. Transamination is funneled through glutamate. This is done through the transfer of the α amino group to α ketoglutarate. Product α-keto acid and glutamate. Alanine to pyruvate and aspartate to oxaloacetate all amino acids except lysine and threonina (lose the α amino through deamination. Glutamate dehydrogenase the oxidative deamination Glutamate only amino acid that undergoes rapid oxidative deamination (glutamate dehydrogenase NH3 + NADH), where amino groups are released as ammonia. After a meal rich in protein the levels of glutamate in liver are increased favoring amino acid degradation and formation of ammonia. Activators of this enzyme are ADP and GDP while ATP and GTP are inhibitors. This reaction occurs in the mitochondria. Ammonia can be transported to the liver from other tissues through two mechanisms that reduce the toxicity of ammonia. One through Glutamine (most tissues through, glutamine synthetase). In the liver the glutamine is converted back to glutamate (Fig. -

ATP Structure and Function
ATP: Universal Currency of Cellular Energy All living things including plants, animals, birds, insects, humans need energy for the proper functioning of cells, tissues and other organ systems. As we are aware that green plants, obtain their energy from the sunlight, and animals get their energy by feeding on these plants. Energy acts as a source of fuel. We, humans, gain energy from the food we eat, but how are the energy produced and stored in our body. A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would result in excessive thermal motion that could damage and then destroy the cell. Rather, a cell must be able to handle that energy in a way that enables the cell to store energy safely and release it for use only as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP). ATP is often called the “energy currency” of the cell, and, like currency, this versatile compound can be used to fill any energy need of the cell. How? It functions similarly to a rechargeable battery. When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. The energy is used to do work by the cell, usually by the released phosphate binding to another molecule, activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to move the contractile muscle proteins. Recall the active transport work of the sodium-potassium pump in cell membranes. -

Transamination in Tumors, Fetal Tissues, and Regenerating Liver* Philip P
Transamination in Tumors, Fetal Tissues, and Regenerating Liver* Philip P. Cohen, M.D., and G. Leverne Hekhuis (From the Laboratory o~ Physiological Chemistry, Yale University School of Medicine, New Haven, Connecticut) (Received for publication June 9, I94I) von Euler and co-workers (22), on the basis of TISSUE SOURCES results of essentially qualitative experiments, first re- Tumors.--The mouse tumors employed in this in- ported that the transaminase activity ot~ tumors was vestigation were the following: low. These investigators, using Jensen sarcoma and normal muscle, measured the rate of disappearance of I. United States Public Health Service No. ~7-- oxaloacetic acid in the following reaction: originally described as a neuroepithelioma (20). 1 2. Sarcoma 37 .1 3. Yale No. xuan estrogen-induced ,) 1( + )-glutamic acid + oxaloacetic acid a mammary adenocarcinoma (5). 1 4. No. x5o9I-A - ~'b a-ketoglutaric acid + aspartic acid spontaneous mammary medullary adenocarcinoma In addition to studies of reaction I in tumors, Braun- (6) "1 5- No. 42--glioblastoma multiforme. 2 6. No. stein and Azarkh (2) studied the reaction: i o8--rhabdomyosarcoma. 2 The tumors were transplanted at regular intervals 2) glutamic acid+a-keto acid to insure a uniform supply. a-ketoglutaric acid + amino acid "-b- Fetal, kitten, and adult cat tissues.--Two pregnant cats provided the fetal tissue. The length of the preg- The amino acids investigated in the case of reaction nancy was uncertain, but was estimated to be in the 2b were the d- and l-forms of alanine, valine, leucine, last trimester in both instances. Hemihysterectomies and isoleucine. The rate of reaction za in which both were performed under nembutal anesthesia and 2 d(--)- and l(+)-glutamic acid were used, plus fetuses removed from each animal. -

Laboratory Diagnostic Approaches in Metabolic Disorders
470 Review Article on Inborn Errors of Metabolism Page 1 of 14 Laboratory diagnostic approaches in metabolic disorders Ruben Bonilla Guerrero1, Denise Salazar2, Pranoot Tanpaiboon2,3 1Formerly Quest Diagnostics, Inc., Ruben Bonilla Guerrero, Rancho Santa Margarita, CA, USA; 2Quest Diagnostics, Inc., Denise Salazar and Pranoot Tanpaiboon, San Juan Capistrano, CA, USA; 3Genetics and Metabolism, Children’s National Rare Disease Institute, Washington, DC, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: R Bonilla Guerrero; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Ruben Bonilla Guerrero. Formerly Quest Diagnostics, Inc., Ruben Bonilla Guerrero, 508 Sable, Rancho Santa Margarita, CA 92688, USA. Email: [email protected]. Abstract: The diagnosis of inborn errors of metabolism (IEM) takes many forms. Due to the implementation and advances in newborn screening (NBS), the diagnosis of many IEM has become relatively easy utilizing laboratory biomarkers. For the majority of IEM, early diagnosis prevents the onset of severe clinical symptoms, thus reducing morbidity and mortality. However, due to molecular, biochemical, and clinical variability of IEM, not all disorders included in NBS programs will be detected and diagnosed by screening alone. This article provides a general overview and simplified guidelines for the diagnosis of IEM in patients with and without an acute metabolic decompensation, with early or late onset of clinical symptoms. The proper use of routine laboratory results in the initial patient assessment is also discussed, which can help guide efficient ordering of specialized laboratory tests to confirm a potential diagnosis and initiate treatment as soon as possible. -

Lecture 11 - Biosynthesis of Amino Acids
Lecture 11 - Biosynthesis of Amino Acids Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction Biosynthetic pathways for amino acids, Text nucleotides and lipids are very old Biosynthetic (anabolic) pathways share common intermediates with the degradative (catabolic) pathways. The amino acids are the building blocks for proteins and other nitrogen-containing compounds 2 2 Introduction Nitrogen Fixation Text Reducing atmospheric N2 to NH3 Amino acid biosynthesis pathways Regulation of amino acid biosynthesis. Amino acids as precursors to other biological molecules. e.g., Nucleotides and porphoryns 3 3 Introduction Nitrogen fixation is carried out by a few Text select anaerobic micororganisms The carbon backbones for amino acids come from glycolysis, the citric acid cycle and the pentose phosphate pathway. The L–stereochemistry is enforced by transamination of α–keto acids 4 4 1. Nitrogen Fixation Microorganisms use ATP and ferredoxin to Text reduce atmospheric nitrogen to ammonia. 60% of nitrogen fixation is done by these microorganisms 15% of nitrogen fixation is done by lighting and UV radiation. 25% by industrial processes Fritz Habers (500°C, 300!atm) N2 + 3 H2 2 N2 5 5 1. Nitrogen Fixation Enzyme has both a reductase and a Text nitrogenase activity. 6 6 1.1 The Reductase (Fe protein) Contains a 4Fe-4S Text center Hydrolysis of ATP causes a conformational change that aids the transfer of the electrons to the nitrogenase domain (MoFe protein) 7 7 1.1 The Nitrogenase (MoFe Protein) The nitrogenase Text component is an α2β2 tetramer (240#kD) Electrons enter the P-cluster 8 8 1.1 The Nitrogenase (MoFe Protein) An Iron-Molybdenum cofactor for the Text nitrogenase binds and reduces the atmospheric nitrogen. -

Amino Acid Catabolism: Urea Cycle the Urea Bi-Cycle Two Issues
BI/CH 422/622 OUTLINE: OUTLINE: Protein Degradation (Catabolism) Digestion Amino-Acid Degradation Inside of cells Urea Cycle – dealing with the nitrogen Protein turnover Ubiquitin Feeding the Urea Cycle Activation-E1 Glucose-Alanine Cycle Conjugation-E2 Free Ammonia Ligation-E3 Proteosome Glutamine Amino-Acid Degradation Glutamate dehydrogenase Ammonia Overall energetics free Dealing with the carbon transamination-mechanism to know Seven Families Urea Cycle – dealing with the nitrogen 1. ADENQ 5 Steps 2. RPH Carbamoyl-phosphate synthetase oxidase Ornithine transcarbamylase one-carbon metabolism Arginino-succinate synthetase THF Arginino-succinase SAM Arginase 3. GSC Energetics PLP uses Urea Bi-cycle 4. MT – one carbon metabolism 5. FY – oxidases Amino Acid Catabolism: Urea Cycle The Urea Bi-Cycle Two issues: 1) What to do with the fumarate? 2) What are the sources of the free ammonia? a-ketoglutarate a-amino acid Aspartate transaminase transaminase a-keto acid Glutamate 1 Amino Acid Catabolism: Urea Cycle The Glucose-Alanine Cycle • Vigorously working muscles operate nearly anaerobically and rely on glycolysis for energy. a-Keto acids • Glycolysis yields pyruvate. – If not eliminated (converted to acetyl- CoA), lactic acid will build up. • If amino acids have become a fuel source, this lactate is converted back to pyruvate, then converted to alanine for transport into the liver. Excess Glutamate is Metabolized in the Mitochondria of Hepatocytes Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. (deaminating) (N,Q,H,S,T,G,M,W) OAA à Asp Glutamine Synthetase This costs another ATP, bringing it closer to 5 (N,Q,H,S,T,G,M,W) 29 N 2 Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. -

Transamination What Is Transamination? Subhadipa 2020 • Important Method of Nitrogen Metabolism of Amino Acids
Subhadipa 2020 Transamination What is Transamination? Subhadipa 2020 • Important method of nitrogen metabolism of amino acids. • Transamination is the transfer of an amine group from an amino acid to a keto acid (amino acid without an amine group), thus creating a new amino acid and keto acid. • Transamination reactions combine reversible amination and deamination, and they mediate redistribution of amino groups among amino acids. • Transaminases (aminotransferases) are widely distributed in human tissues and are particularly active in heart muscle, liver, skeletal muscle, and kidney. • The general reaction of transamination is: Concerned enzyme • The reaction is catalyzed by transaminase or amino transferase. • Enzymes act on L-amino acid but not on D-isomers. • They occur both mitochondria and cytosol as separate enzyme. • There are many transferases, each acts on a particular amino acid and a particular keto acid. Amino and keto acid • All naturally occurring amino acids undergo transamination. • Exceptions include basic amino acids lysine, hydroxyl amino acids, serine and threonine and heterocyclic amino acids proline and hydroxyl-proline. • Keto acids like pyruvic acid, oxaloacetic acid and α- ketoglutaric acid are commonly involved. • Glyoxylate and glutamic γ semialdehyde may also act as amino- receptors in transamination. E-PLP Complex Subhadipa 2020 All transaminase reactions have the same mechanism and use pyridoxal phosphate (a derivative of vitamin B6). Pyridoxal phosphate is linked to the enzyme by formation of a Schiff base between its aldehyde group and the ε-amino group of a specific lysyl residue at the active site and held noncovalently through its positively charged nitrogen atom and the negatively charged phosphate group.