Metabolism Citric Acid Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibolic Nature of Krebs Cycle
Amphibolic nature of Krebs Cycle How what we are is what we eat • In aerobic organisms, the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. • Since the citric acid does both synthesis (anabolic) and breakdown (catabolic) activities, it is called an amphibolic pathway • The citric acid cycle is amphibolic (i.e it is both anabolic and catabolic in its function). • It is said to be an AMPHIBOLIC pathway, because it functions in both degradative or catabolic and biosynthetic or anabolic reactions (amphi = both) A central metabolic pathway or amphibolic pathway is a set of reactions which permit the interconversion of several metabolites, and represents the end of the catabolism and the beginning of anabolism • The KREBS CYCLE or citric acid cycle is a series of reactions that degrades acetyl CoA to yield carbon dioxide, and energy, which is used to produce NADH, H+ and FADH. • The KREBS CYCLE connects the catabolic pathways that begin with the digestion and degradation of foods in stages 1 and 2 with the oxidation of substrates in stage 3 that generates most of the energy for ATP synthesis. • The citric acid cycle is the final common pathway in the oxidation of fuel molecules. In stage 3 of metabolism, citric acid is a final common catabolic intermediate in the form of acetylCoA. • This is why the citric acid cycle is called a central metabolic pathway. Anaplerosis and Cataplerosis Anaplerosis is a series of enzymatic reactions in which metabolic intermediates enter the citric acid cycle from the cytosol. Cataplerosis is the opposite, a process where intermediates leave the citric acid cycle and enter the cytosol. -

Development of a System of High Ornithine and Citrulline Production by a Plant-Derived Lactic Acid Bacterium, Weissella Confusa K-28
Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28 㸦᳜≀⏤᮶ங㓟⳦ :HLVVHOODFRQIXVD. ࠾ࡅࡿ ࢜ࣝࢽࢳࣥཬࡧࢩࢺࣝࣜࣥ㧗⏕⏘ࢩࢫࢸ࣒ࡢᵓ⠏㸧 Md Rakhimuzzaman Student ID: D164180 Supervisor: Prof. Masanori Sugiyama Department of Probiotic Science for Preventive Medicine Graduate School of Biomedical and Health Sciences Hiroshima University, Japan 2019 ABBREVIATIONS ADI arginine deiminase AUC area under curve BLAST basic local alignment search tool CK carbamate kinase dNTP deoxyribonucleotide triphosphate DDBJ DNA Data Bank of Japan EDTA ethylenediamine tetra-acetic acid EtOH ethanol EPS exopolysaccharide GABA gamma-aminobutyric acid GRAS generally recognized as safe HPLC high performance liquid chromatography LAB lactic acid bacteria Lb. Lactobacillus OD optical density OTC ornithine carbamoyltransferase PITC phenyl isothiocyanate TAE tris-acetate-ethylenediamine tetra-acetic acid TE tris-ethylenediamine tetra-acetic acid TEA triethylamine Tris tris(hydroxymethyl)aminomethane rDNA ribosomal deoxyribonucleic acid RT-PCR reverse transcription polymerase chain reaction CONTENTS INTRODUCTION 1-17 OBJECTIVES 18 MATERIALS AND METHODS 19-34 RESULTS 35-57 DISCUSSIONS 58-65 CONCLUSION 66-67 ACKNOWLEDGEMENTS 68 REFERENCES 69-84 INTRODUCTION Lactic acid bacteria (LAB) are an order of gram-positive, acid-tolerant, generally nonsporulating, non-respiring, either rod-shaped or spherical microorganisms. Since LAB lack the ability of synthesizing porphyrins (heme, and components of respiratory chains) (Pessione et al, 2010), the bacteria can not generate ATP without external heme supplementation (Pessione et al, 2010). The LAB strains can only obtain ATP by fermentation, usually they generate the ATP from some sugars. Because LAB do not use oxygen for energy production, the bacteria easily grow under anaerobic conditions, but they can also grow in presence of oxygen. -

Exam #2 Review
Exam #2 Review Exam #2 will cover all the material that has been presented in class since Exam #1 and up through the metabolism introduction. This includes eukaryotic cell structure / function, transport, the closed system growth curve, enzymes and the introduction to metabolism. As always, it is best to begin by studying your notes and then after you feel your study is complete, take some time to look through this review. I. Eukaryotic cell structure / function A. There is a great deal of variance among eukaryotic cells - from protozoan cells to yeast cells to human cells. Fungi and protists (classically split into algae and protozoa) are eukaryotic representatives of the microbial world. B. Structure of the eukaryotic cell. 1. Cytoskeleton - provides structure and shape of cell, three components: a. Microtubules - largest element of cytoskeleton, composed of hollow cylinders of tubulin, form mitotic spindles, cilia and flagella and cell “highways”. b. Microfilaments - smallest element of cytoskeleton, composed of actin, involved in motion (pseudopod formation). c. Intermediate filaments - very stable structural element, play a supportive role, composed of proteins including keratin. Practice: Microfilaments a. are a component of the cytoskeleton. b. are long, twisted polymers of a protein called actin. c. form eukaryotic flagella. d. are made of tubulin. e. a and b f. c and d 2. Nucleus a. Bound by both an inner and outer membrane. The space between the two membranes is called the perinuclear space. The membrane has large nuclear pores through which proteins can pass (Why is this important?) b. Linear pieces of DNA are packaged by wrapping one and three quarters times around a histone octamer to form a core particle. -

Metabolic Reprogramming in Prostate Cancer
www.nature.com/bjc REVIEW ARTICLE Metabolic reprogramming in prostate cancer Fahim Ahmad 1,2, Murali Krishna Cherukuri2 and Peter L. Choyke1 Although low risk localised prostate cancer has an excellent prognosis owing to effective treatments, such as surgery, radiation, cryosurgery and hormone therapy, metastatic prostate cancer remains incurable. Existing therapeutic regimens prolong life; however, they are beset by problems of resistance, resulting in poor outcomes. Treatment resistance arises primarily from tumour heterogeneity, altered genetic signatures and metabolic reprogramming, all of which enable the tumour to serially adapt to drugs during the course of treatment. In this review, we focus on alterations in the metabolism of prostate cancer, including genetic signatures and molecular pathways associated with metabolic reprogramming. Advances in our understanding of prostate cancer metabolism might help to explain many of the adaptive responses that are induced by therapy, which might, in turn, lead to the attainment of more durable therapeutic responses. British Journal of Cancer https://doi.org/10.1038/s41416-021-01435-5 BACKGROUND Several tools have been designed to assess metabolism without Cellular metabolism involves complex biochemical processes disturbing the system. Advances in nuclear magnetic resonance through which specific nutrients are consumed. Carbohydrates, and mass spectroscopy have helped to quantify the carbon influx fatty acids and amino acids are the main source of nutrients for of the central metabolic pathways (e.g. TCA cycle, glycolysis and energy homeostasis and macromolecular synthesis. They are the pentose phosphate pathway) in mammalian systems, and this main constituent of core metabolic pathways that can be approach has been instrumental in demonstrating that the classified as anabolic, catabolic or waste producing (Fig. -

KINETICS of HYDROLYSIS of ACETIC ANHYDRIDE by IN-SITU FTIR SPECTROSCOPY an Experiment for the Undergraduate Laboratory
.tA... 5-4._l_a_b _o_r._a_t_o_r.:.y________ ) KINETICS OF HYDROLYSIS OF ACETIC ANHYDRIDE BY IN-SITU FTIR SPECTROSCOPY An Experiment for the Undergraduate Laboratory SHAKER HA.JI, CAN ERKEY University of Connecticut • Storrs, CT 06269-3222 he senior-level chemical engineering undergraduate laboratory course at the University of Connecticut con 2 T sists of two four-hour labs per week, during which groups of three to four students typically perform five ex Quite a few studies have been reported in the literature on the 2 periments during the course of the semester. Each experi kinetics of hydrolysis of acetic anhydride. [J, A,5l Eldridge and ment is studied for either one or two weeks, depending on its Piretl41 obtained the pseudo-first-order reaction rate constant complexity and the scale of the equipment. The students are using a batch reactor. To determine the acetic anhydride con given only the general goals for each experiment and are re centration, samples from the reactor were withdrawn into tared quired to define their own objectives, to develop an experi flasks containing 15-20 times the quantity of saturated aniline mental plan, to prepare a pre-lab report (including a discus water required to react with the sample. Since the anhydride sion of safety measures), to perform the experiments and ana rapidly acetylates the aniline, producing acetanilide and ace lyze the data, and to prepare group or individual written and/ tic acid, the samples were then titrated to determine the con or oral reports. centration of acetic acid. In another study, Shatyski and One or two of the experiments in this course involve reac Hanesianrsi determined the kinetics of the above reaction by tion kinetics. -

Citric Acid Cycle
CHEM464 / Medh, J.D. The Citric Acid Cycle Citric Acid Cycle: Central Role in Catabolism • Stage II of catabolism involves the conversion of carbohydrates, fats and aminoacids into acetylCoA • In aerobic organisms, citric acid cycle makes up the final stage of catabolism when acetyl CoA is completely oxidized to CO2. • Also called Krebs cycle or tricarboxylic acid (TCA) cycle. • It is a central integrative pathway that harvests chemical energy from biological fuel in the form of electrons in NADH and FADH2 (oxidation is loss of electrons). • NADH and FADH2 transfer electrons via the electron transport chain to final electron acceptor, O2, to form H2O. Entry of Pyruvate into the TCA cycle • Pyruvate is formed in the cytosol as a product of glycolysis • For entry into the TCA cycle, it has to be converted to Acetyl CoA. • Oxidation of pyruvate to acetyl CoA is catalyzed by the pyruvate dehydrogenase complex in the mitochondria • Mitochondria consist of inner and outer membranes and the matrix • Enzymes of the PDH complex and the TCA cycle (except succinate dehydrogenase) are in the matrix • Pyruvate translocase is an antiporter present in the inner mitochondrial membrane that allows entry of a molecule of pyruvate in exchange for a hydroxide ion. 1 CHEM464 / Medh, J.D. The Citric Acid Cycle The Pyruvate Dehydrogenase (PDH) complex • The PDH complex consists of 3 enzymes. They are: pyruvate dehydrogenase (E1), Dihydrolipoyl transacetylase (E2) and dihydrolipoyl dehydrogenase (E3). • It has 5 cofactors: CoASH, NAD+, lipoamide, TPP and FAD. CoASH and NAD+ participate stoichiometrically in the reaction, the other 3 cofactors have catalytic functions. -

Amino Acid Degradation; Urea Cycle
Amino acid degradation; urea cycle Kristina Mlinac Jerković [email protected] In animals, amino acids undergo oxidative degradation in three different metabolic circumstances: 1. During the normal synthesis and degradation of cellular proteins, some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. 2. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. 3. During starvation or in uncontrolled diabetes mellitus, when carbohydrates are either unavailable or not properly utilized, cellular proteins are used as fuel. Metabolic fates of amino groups - The metabolism of nitrogen-containing molecules such as proteins and nucleic acids differs significantly from that of carbohydrates and lipids. - Whereas the latter molecules can be stored and mobilized as needed for biosynthetic reactions or for energy generation, there is no nitrogen-storing molecule (one exception to this rule is storage protein in seeds). - Organisms must constantly replenish their supply of usable nitrogen to replace organic nitrogen that is lost in catabolism. For example, animals must have a steady supply of amino acids in their diets to replace the nitrogen excreted as urea, uric acid, and other nitrogenous waste products. - Since excess dietary amino acids are neither stored for future use nor excreted, they are converted to common metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-keto-glutarate. - Consequently, amino acids are also precursors of glucose, fatty acids, and ketone bodies and are therefore metabolic fuels. -

Protein Metabolism and It's Disorders
Protein Metabolism and it’s Disorders (Course : M.Sc. Zoology) Course Code: ZOOL 4008 (Biochemistry and Metabolism), Semester –II Dr. Shyam Babu Prasad Assistant Professor Department of Zoology Mahatma Gandhi Central University (MGCU), Motihari-845401 (Bihar) Email: [email protected] Important facts of the Protein Metabolism •Proteins metabolism referred as synthesis of protein from Amino acids (Anabolism) and breakdown of proteins (Catabolism). •In the Unit II, we discussed the synthesis (Anabolism) of various form of the proteins from different amino acids. • Here we mainly focused on the Catabolism of the protein, which is integral part of metabolism of the nitrogen-containing molecules. • Nitrogen enters in to the body in various form of compounds that is present in food, the most important is amino acids (as dietary food). • During Protein/amino acid metabolism (Catabolism), Nitrogen leaves the body as urea, ammonia, and in form of other derivatives. • The Catabolism of the protein depends on the amino acid pool and protein turnover (the rate of protein synthesis and degradation is equal). • The amino acids present in through out of the body (cells, blood, and the extracellular fluids etc) is called amino acid pools. It is maintained by following factors as represented in below diagram: Body protein Dietary degradation Protein 400gm/day 600gm/day Supplied Amino acid pool Depleted Synthesis of Remaining Synthesis of Glucose, Synthesis of amino acids Nitrogen containing Glycogen, the Body burned as compound like Ketone Proteins -

Biotransformation: Basic Concepts (1)
Chapter 5 Absorption, Distribution, Metabolism, and Elimination of Toxics Biotransformation: Basic Concepts (1) • Renal excretion of chemicals Biotransformation: Basic Concepts (2) • Biological basis for xenobiotic metabolism: – To convert lipid-soluble, non-polar, non-excretable forms of chemicals to water-soluble, polar forms that are excretable in bile and urine. – The transformation process may take place as a result of the interaction of the toxic substance with enzymes found primarily in the cell endoplasmic reticulum, cytoplasm, and mitochondria. – The liver is the primary organ where biotransformation occurs. Biotransformation: Basic Concepts (3) Biotransformation: Basic Concepts (4) • Interaction with these enzymes may change the toxicant to either a less or a more toxic form. • Generally, biotransformation occurs in two phases. – Phase I involves catabolic reactions that break down the toxicant into various components. • Catabolic reactions include oxidation, reduction, and hydrolysis. – Oxidation occurs when a molecule combines with oxygen, loses hydrogen, or loses one or more electrons. – Reduction occurs when a molecule combines with hydrogen, loses oxygen, or gains one or more electrons. – Hydrolysis is the process in which a chemical compound is split into smaller molecules by reacting with water. • In most cases these reactions make the chemical less toxic, more water soluble, and easier to excrete. Biotransformation: Basic Concepts (5) – Phase II reactions involves the binding of molecules to either the original toxic molecule or the toxic molecule metabolite derived from the Phase I reactions. The final product is usually water soluble and, therefore, easier to excrete from the body. • Phase II reactions include glucuronidation, sulfation, acetylation, methylation, conjugation with glutathione, and conjugation with amino acids (such as glycine, taurine, and glutamic acid). -
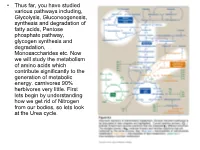
Transaminations and Urea Cycle
• Thus far, you have studied various pathways including, Glycolysis, Gluconeogenesis, synthesis and degradation of fatty acids, Pentose phosphate pathway, glycogen synthesis and degradation, Monosaccharides etc. Now we will study the metabolism of amino acids which contribute significantly to the generation of metabolic energy. carnivores 90% herbivores very little. First lets begin by understanding how we get rid of Nitrogen from our bodies, so lets look at the Urea cycle. 1 α Amino group keeps amino acids safe from oxidative breakdown. Removal of this group is essential for energy production it is an obligatory step for catabolism. Nitrogen can then be incorporated or excreted.This is done through transamination or oxidative deamination, provide amonia and aspartate sources for the Urea cycle. Transamination is funneled through glutamate. This is done through the transfer of the α amino group to α ketoglutarate. Product α-keto acid and glutamate. Alanine to pyruvate and aspartate to oxaloacetate all amino acids except lysine and threonina (lose the α amino through deamination. Glutamate dehydrogenase the oxidative deamination Glutamate only amino acid that undergoes rapid oxidative deamination (glutamate dehydrogenase NH3 + NADH), where amino groups are released as ammonia. After a meal rich in protein the levels of glutamate in liver are increased favoring amino acid degradation and formation of ammonia. Activators of this enzyme are ADP and GDP while ATP and GTP are inhibitors. This reaction occurs in the mitochondria. Ammonia can be transported to the liver from other tissues through two mechanisms that reduce the toxicity of ammonia. One through Glutamine (most tissues through, glutamine synthetase). In the liver the glutamine is converted back to glutamate (Fig. -
Pyruvate Dehydrogenase
Aerobic Metabolism I: The Citric Acid Cycle Chapter 9 Overview n Live processes - series of oxidation-reduction reactions ¨ Ingestion of proteins, carbohydrates, lipids ¨ Provide basic building blocks for major molecules ¨ Produces energy n Aerobic metabolism I ¨ Citric Acid Cycle – series of reactions that release chemical energy stored in acetyl-CoA n Acetyl-CoA derived from pyruvate From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Chapter 9: Overview §Citric acid cycle §Two-carbon fragments oxidized to form CO2 + §NAD /FAD reduced to NADH/FADH2 §Electron transport chain §Transfer of electrons from NADH/FADH2 to electron carriers §Terminal acceptor O2 §Oxidative phosphorylation §Energy released forms proton gradient §Drives ATP synthesis Figure 9.1 Overview of Aerobic Metabolism From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Section 9.1: Oxidation-Reduction Reactions Figure 9.3 Reduction of Pyruvate by NADH §Redox reactions – electron transfer between an electron donor (reducing agent) & electron acceptor (oxidizing agent) §Many redox reactions have both an electron (e-) and a proton (H+) transferred §Conversion of pyruvate and NADH to lactate and NAD+ (shown above) is under anaerobic conditions From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Section 9.1: Oxidation-Reduction Reactions §Half-reactions of redox reactions Cu loses e-, electron donor Cu+ ß à Cu2+ + e- Fe gains e-, electron acceptor Fe3+ + e- ß à Fe2+ Figure 9.4 An Electrochemical -

Two-Stage, Dilute Sulfuric Acid Hydrolysis of Hardwood for Ethanol Production
TWO-STAGE, DILUTE SULFURIC ACID HYDROLYSIS OF HARDWOOD FOR ETHANOL PRODUCTION John F. Harris, B.S. Chemical Engineer Andrew J. Baker, B.S. Chemical Engineer John I. Zerbe, Ph.D. Program Manager Energy Research, Development, and Application Forest Products Laboratory, Forest Service, USDA Madison, Wisconsin53705 ABSTRACT The Forest Products Laboratory has developed fundamental kinetic relationships for the hydrolysis of lignocellulose dating back to World War II. Recent work at the Laboratory has provided additional informa tion on hydrolysis of red oak by a two-stage process at higher tempera tures with lower liquid-to-solid ratios. The complications of working with mixtures of sugars and acetic acid (6 percent of oak) are such that effective fractionation is necessary. This is best accomplished by prehydrolysis using dilute sulfuric acid. Dilute acid hydrolysis is also currently the best method for conversion of the residual prehydro lyzed lignocellulose to glucose. The course and yield of reactions are well predicted by kinetics data, but more attention needs to be given to the effects of ash constituents on the catalyst acid and the rever sion reaction, and in reducing the water-to-wood ratio in hydrolysis. The purity of the solutions generated by the two-stage process is considerably better than that obtained by the previous percolation process, but this advantage is offset somewhat by the higher yields of the percolation process. Projections on the economics of the two-stage process, although better than for processes based on prior technology, are still considered high risk. 1151 TWO-STAGE, DILUTE SULFURIC ACID HYDROLYSIS OF HARDWOOD FOR ETHANOL PRODUCTION INTRODUCTION Interest in wood hydrolysis dates to 1819 when Braconnot dis covered that cellulose could be dissolved in concentrated acid solutions and converted to sugar.