Formation, Maturation, and Disorders of White Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Connexions of the Amygdala
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.2.137 on 1 April 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 137 The connexions of the amygdala W. M. COWAN, G. RAISMAN, AND T. P. S. POWELL From the Department of Human Anatomy, University of Oxford The amygdaloid nuclei have been the subject of con- to what is known of the efferent connexions of the siderable interest in recent years and have been amygdala. studied with a variety of experimental techniques (cf. Gloor, 1960). From the anatomical point of view MATERIAL AND METHODS attention has been paid mainly to the efferent connexions of these nuclei (Adey and Meyer, 1952; The brains of 26 rats in which a variety of stereotactic or Lammers and Lohman, 1957; Hall, 1960; Nauta, surgical lesions had been placed in the diencephalon and and it is now that there basal forebrain areas were used in this study. Following 1961), generally accepted survival periods of five to seven days the animals were are two main efferent pathways from the amygdala, perfused with 10 % formol-saline and after further the well-known stria terminalis and a more diffuse fixation the brains were either embedded in paraffin wax ventral pathway, a component of the longitudinal or sectioned on a freezing microtome. All the brains were association bundle of the amygdala. It has not cut in the coronal plane, and from each a regularly spaced generally been recognized, however, that in studying series was stained, the paraffin sections according to the Protected by copyright. the efferent connexions of the amygdala it is essential original Nauta and Gygax (1951) technique and the frozen first to exclude a contribution to these pathways sections with the conventional Nauta (1957) method. -

Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly
333 Apparent Atypical Callosal Dysgenesis: Analysis of MR Findings in Six Cases and Their Relationship to Holoprosencephaly A. James Barkovich 1 The MR scans of six pediatric patients with apparent atypical callosal dysgenesis (presence of the dorsal corpus callosum in the absence of a rostral corpus callosum) were critically analyzed and correlated with developmental information in order to assess the anatomic, embryologic, and developmental implications of this unusual anomaly. Four patients had semilobar holoprosencephaly; the dorsal interhemispheric commis sure in these four infants resembled a true callosal splenium. All patients in this group had severe developmental delay. The other two patients had complete callosal agenesis with an enlarged hippocampal commissure mimicking a callosal splenium; both were developmentally and neurologically normal. The embryologic implications of the pres ence of these atypical interhemispheric connections are discussed. Differentiation between semilobar holoprosencephaly and agenesis of the corpus callosum with enlarged hippocampal commissure-two types of apparent atypical callosal dysgenesis-can be made by obtaining coronal, short TR/TE MR images through the frontal lobes. Such differentiation has critical prognostic implications. AJNR 11:333-339, March{Apri11990 Abnormalities of the corpus callosum are frequently seen in patients with con genital brain malformations [1-5); a recent publication [5) reports an incidence of 47%. The corpus callosum normally develops in an anterior to posterior direction. The genu forms first, followed by the body, splenium, and rostrum. Dysgenesis of the corpus callosum is manifested by the presence of the earlier-formed segments (genu , body) and absence of the later-formed segments (splenium, rostrum) [4-6]. We have recently encountered six patients with findings suggestive of atypical callosal dysgenesis in whom there was apparent formation of the callosal splenium in the absence of the genu and body. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Rhesus Monkey Brain Atlas Subcortical Gray Structures
Rhesus Monkey Brain Atlas: Subcortical Gray Structures Manual Tracing for Hippocampus, Amygdala, Caudate, and Putamen Overview of Tracing Guidelines A) Tracing is done in a combination of the three orthogonal planes, as specified in the detailed methods that follow. B) Each region of interest was originally defined in the right hemisphere. The labels were then reflected onto the left hemisphere and all borders checked and adjusted manually when necessary. C) For the initial parcellation, the user used the “paint over function” of IRIS/SNAP on the T1 template of the atlas. I. Hippocampus Major Boundaries Superior boundary is the lateral ventricle/temporal horn in the majority of slices. At its most lateral extent (subiculum) the superior boundary is white matter. The inferior boundary is white matter. The anterior boundary is the lateral ventricle/temporal horn and the amygdala; the posterior boundary is lateral ventricle or white matter. The medial boundary is CSF at the center of the brain in all but the most posterior slices (where the medial boundary is white matter). The lateral boundary is white matter. The hippocampal trace includes dentate gyrus, the CA3 through CA1 regions of the hippocamopus, subiculum, parasubiculum, and presubiculum. Tracing A) Tracing is done primarily in the sagittal plane, working lateral to medial a. Locate the most lateral extent of the subiculum, which is bounded on all sides by white matter, and trace. b. As you page medially, tracing the hippocampus in each slice, the superior, anterior, and posterior boundaries of the hippocampus become the lateral ventricle/temporal horn. c. Even further medially, the anterior boundary becomes amygdala and the posterior boundary white matter. -

The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1935 The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat James K. L. Choy Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Anatomy Commons Recommended Citation Choy, James K. L., "The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat" (1935). Master's Theses. 22. https://ecommons.luc.edu/luc_theses/22 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1935 James K. L. Choy LOYOLA UNIVERSITY SCHOOl, OF MEDICINE THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. A THESIS SUBMITTED TO THE FACULTY of the GRADUATE SCHOOL of LOYOLA UNIVERSITY IN CANDIDACY FOR THE DEGREE OF MASTER OF SCIENCE by James K.L. Choy, B.S.M. 1935 THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. I. PREFACE Before entering upon a discussion of the problem itself, I would lil{e to take this opportunity to acknowledge the assis tance and encouragement I received in the preparation of this paper. To Dr. R. M. Strong, who suggested the problem, I am deeply obligated for his encouragement, practical guidance, and helpful suggestions in the procedure of this work. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

Microsurgical Anatomy of the Anterior Commissure Through the Anterior
J Surg Med. 2020;4(10):853-856. Research article DOI: 10.28982/josam.813998 Araştırma makalesi Microsurgical anatomy of the anterior commissure through the anterior interhemispheric transcallosal approach to the third ventricle: An anatomical and morphological study Üçüncü ventriküle anterior interhemisferik transkallozal yaklaşım yoluyla anterior komissürün mikrocerrahi anatomisi: Anatomik ve morfolojik bir çalışma Seçkin Aydın 1, Ayşegül Esen Aydın 2, Necmettin Tanrıöver 3 1 University of Health Sciences, Okmeydani Abstract Training and Research Hospital, Department of Aim: The third ventricle is a funnel-shaped cavity located deep in the brain and difficult to access with surgical approach. The anterior Neurosurgery, Sisli, Istanbul, Turkey commissure is an anatomical structure located on the anterior wall of the third ventricle. This study aimed to demonstrate the use of the 2 University of Health Sciences, Bakirkoy Prof. Dr. Mazhar Osman Training and Research Hospital anterior interhemispheric transcallosal approaches to access the third ventricle, evaluate the microsurgical anatomy of the anterior for Psychiatric, Neurologic and Neurosurgical commissure and investigate the morphological features of this region. Diseases, Department of Neurosurgery, Bakirkoy, Methods: Eleven cadaveric brain specimens were dissected using microsurgical tools. Different anterior interhemispheric routes to the Istanbul, Turkey third ventricle were demonstrated, and stepwise dissections were performed to expose the limbs of the anterior commissure. 3 -

White Matter Dissection and Structural Connectivity of the Human Vertical
www.nature.com/scientificreports OPEN White matter dissection and structural connectivity of the human vertical occipital fasciculus to link vision-associated brain cortex Tatsuya Jitsuishi1, Seiichiro Hirono2, Tatsuya Yamamoto1,3, Keiko Kitajo1, Yasuo Iwadate2 & Atsushi Yamaguchi1* The vertical occipital fasciculus (VOF) is an association fber tract coursing vertically at the posterolateral corner of the brain. It is re-evaluated as a major fber tract to link the dorsal and ventral visual stream. Although previous tractography studies showed the VOF’s cortical projections fall in the dorsal and ventral visual areas, the post-mortem dissection study for the validation remains limited. First, to validate the previous tractography data, we here performed the white matter dissection in post-mortem brains and demonstrated the VOF’s fber bundles coursing between the V3A/B areas and the posterior fusiform gyrus. Secondly, we analyzed the VOF’s structural connectivity with difusion tractography to link vision-associated cortical areas of the HCP MMP1.0 atlas, an updated map of the human cerebral cortex. Based on the criteria the VOF courses laterally to the inferior longitudinal fasciculus (ILF) and craniocaudally at the posterolateral corner of the brain, we reconstructed the VOF’s fber tracts and found the widespread projections to the visual cortex. These fndings could suggest a crucial role of VOF in integrating visual information to link the broad visual cortex as well as in connecting the dual visual stream. Te VOF is the fber tract that courses vertically at the posterolateral corner of the brain. Te VOF was histori- cally described in monkey by Wernicke1 and then in human by Obersteiner2. -
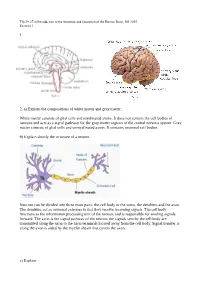
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Study Skills Workshop: Great Ways to Study
Learning & Academic Resources Department/Providing Pathways to Academic Success Study Skills Workshop: Great Ways to Study This video will focus on 3 textbook study techniques. The first one relates to how you should leverage a chapter summary. The second technique will discuss how you can Read in Layers to learn the information in a more efficient manner. Finally, we will address when you should outline a chapter and how to do that. When Scott distributes the first handout go to the next page and follow along. [Rev. 10/2020] 1 Learning & Academic Resources Department/Providing Pathways to Academic Success Summary and Conclusions Summaries Study Reading Method 23 BLANKS From Politics in America, 3rd Edition, By Lance T. Leloup. St. Paul: West Publishing Company, 1991. P. 381 1. Throughout most of the nation’s first century, 7. Presidents have been most successful in national politics was dominated by _______. securing congressional approval in the areas of Occasionally, the pendulum swung towards the ________ affairs and national _______ followed presidency, as in the era of _______and by social welfare and agriculture. Presidents have _______. been least successful in getting Congress to approve their proposals in ______________. 2. The balance of power between the president and Congress permanently changed after the 8. Presidents experience ______ influence with administration of Franklin Roosevelt, architect of Congress through their term. This was the _______ presidency. particularly true of Ronald Regan. As a result, presidents must use their limited resources 3. Reacting to the “______ presidency” and to carefully. They must move ______in the first abuses of presidential power, Congress took a year, set clear legislative priorities, hire number of steps in the 1970’s and 1980’s to experienced staff, and understand the needs of _______ its power. -

A Pan-Mammalian Map of Interhemispheric Brain Connections Predates the Evolution of the Corpus Callosum
A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum Rodrigo Suáreza,1, Annalisa Paolinoa, Laura R. Fenlona, Laura R. Morcoma, Peter Kozulina, Nyoman D. Kurniawanb, and Linda J. Richardsa,c,1 aQueensland Brain Institute, The University of Queensland, Brisbane, QLD 4070, Australia; bCentre for Advanced Imaging, The University of Queensland, Brisbane, QLD 4070, Australia; and cSchool of Biomedical Sciences, The University of Queensland, Brisbane, QLD 4070, Australia Edited by Jon H. Kaas, Vanderbilt University, Nashville, TN, and approved August 1, 2018 (received for review May 14, 2018) The brain of mammals differs from that of all other vertebrates, in embryonic astroglia (13), which is exclusively present in euthe- having a six-layered neocortex that is extensively interconnected rians (14). The evolution of the corpus callosum as a distinct within and between hemispheres. Interhemispheric connections are tract allowed a significant expansion of the number of inter- conveyed through the anterior commissure in egg-laying mono- hemispheric neocortical connections in species with large brains tremes and marsupials, whereas eutherians evolved a separate (15). The corpus callosum carries fibers topographically arranged commissural tract, the corpus callosum. Although the pattern of according to the position of their cell bodies (16–18) and con- interhemispheric connectivity via the corpus callosum is broadly nects mostly similar (homotopic) but also different (heterotopic) shared across eutherian species, it is not known whether this pattern regions in each hemisphere (Fig. 1B). However, although the arose as a consequence of callosal evolution or instead corresponds map of callosal fibers in eutherians is well-established, and in- to a more ancient feature of mammalian brain organization.