Temporal Assessment Using WQI of River Henwal, a Tributary of River Ganga in Himalayan Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Initial Environmental Examination IND:Uttarakhand Emergency
Initial Environmental Examination Project Number: 47229-001 December 2014 IND: Uttarakhand Emergency Assistance Project Submitted by Uttarakhand Emergency Assistance Project (Roads & Bridges), Government of Uttarakhand, Dehardun This report has been submitted to ADB by the Program Implementation Unit, Uttarkhand Emergency Assistance Project (R&B), Government of Uttarakhand, Dehradun and is made publicly available in accordance with ADB’s public communications policy (2011). It does not necessarily reflect the views of ADB. STATUS OF COMPLIANCE WITH ADB COMMENTS AND SUGGESTIONS (ADB APPROVAL DATE 15-10-2014) SUB-PROJECT: REHABILITATION OF DAMAGED ROADS UNDER CONTRACT PACKAGES C36, C39, C41, C43, C44 and C45 ROAD SUB-PROJECT S.N. Comments/Suggestions Status of Compliance Remarks 1 We refer to the submission of initial environmental examination (lEE) report for the road sub-projects ……………….. ……………….. in Tehri district proposed for financing under UEAP. The scope includes restoration works (repairs and rehabilitation) of the damaged stretches including resurfacing; repair of pavement overlay; patch work; repairs of protection or retaining walls; repairs and restoration of culverts; and providing all necessary road furniture and roadside safety hardware for the following roads: a) Pratapnagar-Tehri Motor Road (Package No: UEAP/PWD/C36); b) Gaza-Nakot road (Package No: UEAP/PWD/C39); c) Kirtinagar-Dangdhari road (Package No: UEAP/PWD/C41); d) Tehri-Ghansali-Tilwara road (Package No: UEAP/PWD/C43); e) Chamba-Mussoorrie road (Package No: UEAP/PWD/C44); and f) Kirtinagar-Sorakhal road (Package No: UEAP/PWD/C45). 2 We note from the lEE report that the proposed works would be strictly confined within the existing ……………….. ……………….. right-of-way belonging to Public Works Department, Government of Uttarakhand; and no widening or realignment works would be undertaken under the proposed scope. -

Garhwal Zone Name of Sub Substation Ph
Garhwal Zone Name of Sub SubStation Ph. Division name Name of Sub Station Name of Feeder JE NO SDO NO EE NO Division No. V.I.P 9412075900 Haridwar Road PPCL Ajabpur 9412079519 Nahru Colony Araghar Araghar 1352673620 Nehru Colony 9412075901 Paradeground 9412075925 Raphel Home Ordinance Factory Raipur 9412075953 Vidhan Sabha VVIP Tilak Road 9412075914 Vijay Colony Bindal 1352716105 Bindal Dastana Factory 9412056089 Doon School Bindal 9412075907 V.I.P DEHRADUN (CENTER) 9412075974 Yamuna 9412075917 Chakrata Road Govindgarh 1352530137 Kanwali Road Govindgarh Vijay Park 9412075929 Survey Patel Road 9412075912 Ghanta Ghar Nashvilla Road Parade Ground Parade Ground 1352716145 9412075906 Yojna Bhawan Rajpur Road 9412056089 Substation Sachivalaya Tyagi Road Race Course(01352101123) Patel Road Patel Road 0135-2716143 9412075926 9412057016 Lakhi Bagh Dhamawala Kaulagarh Jaspur CCL Anarwala 1352735538 Dehra 9412075918 Bijapur Pump House Jakhan Anarwala 9412075908 VIP Max Hospital Old Mussoorie Dakpatti 0135-2734211 Malsi 9412075915 Doon Vihar VIP K.P.L L.B.S Kunjbhawan 1352632024 Pump Feeder L.C.H Strawferry Bank Kingrange ITBP DEHRADUN (NORTH) Kyarkuli 1352632859 9412075777 Mussoorie Thatyur 9412075923 9412075910 Murray Pump Survey Landore A.I.R (I/F) Landour 1352632017 PPCL Kirkland D.I.W.S Amity Dilaram Bazar NHO Rajpur Road Hathibarakala 1352741327 9412075919 9412075909 Doon Vihar Bari Ghat Hathibarkala Nadi Rispana 9412075920 Chidyamandi 9412075920 Sahastradhara Sahastradhara 1352787262 Ordinance Factory 9412075911 Sahastradhara 9412075913 MDDA -

Copy of Uttarakhand Backpacking
2019 UTTARAKHAND BACKPACKING DELHI-RISHIKESH-AULI-KANATAL-DELHI www.justwravel.com | 9667051161 U T T A R A K H A N D B A C K P A C K I N G UTTARAKHAND: THE LAND OF GODS Uttarakhand is one of those northern states of India whose glory and beauty cannot be described into words. A state Lord Shiva chose to be his home, and gods gave the blessings to be home of the origin of River Ganga, the holiest of all. Uttarakhand, as the name suggests was previously a northern part of another state (Uttar Pradesh) and now has its boundaries with China (Tibet), Nepal, Uttarakhand and Himachal. This year, pack your bags nad see the side of Uttarakhand that you’d have never seen or heard of, The Wraveler Side! Covering some of the most beautiful and famous destinations accessible by road, this backpacking trip to Uttarakhand is going to be a treat for the shutterbug in you. I T I N E R A R Y 6 N / 7 D Day 1 OVERNIGHT TRAVEL FROM DELHI TO RISHIKESH VIA HARIDWAR Approximately 230 kms away from Delhi, Rishikesh is our first pit stop and will be the kick off point of an incredible adventure! The overnight journey would be around 5-6 hours long and you’ll reach Rishikesh in the morning the next day, crossing Haridwar on the way which is a famous tourist spot for its artistic feels and is also a humble city, famous being one of the holiest in our country. Day 2 RISHIKESH LOCAL SIGHTSEEING. -

Annual Progress Report-ILSP 2018-2019
Annual Progress Report-ILSP 2018-2019 Integrated Livelihood Support Project (ILSP) IFAD Loan no.: #856-IN Annual Progress Report Financial Year 2018-19 (1stApril 2018 – 31st March 2019) Central Project Coordination Unit (CPCU) Uttarakhand Gramya Vikas Samiti (UGVS) Project Society Watershed Management Directorate (PSWMD) Uttarakhand Parvatiya Aajeevika Sanvardhan Company (UPASaC) 216, Panditwari, Phase 2, Dehradun Phone & Fax: 0135-2773800/2774800 Email: [email protected] Page 1 of 183 Annual Progress Report-ILSP 2018-2019 Project At a Glance Background of the Project Government of Uttarakhand, with support from the International Fund for Agriculture Development (IFAD), is implementing Integrated Livelihood Support Project (ILSP) since July 2013. ILSP was scheduled to be completed on 31st March 2019 with loan closing on 31st March 2021. The project is being implemented in 44 blocks of 11 hill districts of Uttarakhand i.e. Almora, Bageshwar, Nainital, Champawat, Pithoragarh, Chamoli, Tehri, Uttarkashi, Pauri, Dehradun, and Rudraprayag. Figure 1 Project Area Objectives The overall objective (goal) of ILSP is to reduce poverty in hill districts of Uttarakhand. This is being achieved via more immediate development objective to “enable rural households to take up sustainable livelihood opportunities integrated with the wider economy”. The strategy behind ILSP is to adopt a two-pronged approach to build livelihoods in hill districts. The first of these is to support and develop the Page 2 of 183 Annual Progress Report-ILSP 2018-2019 food production systems, which remain the main means of support for most households. The second main thrust of the project is to generate cash incomes by supporting non-farm livelihoods, especially community involvement in rural tourism, and vocational training. -

A Private Heaven Imaging Living in a Farm Surrounded by a Garden Full of Colourful Flowers and Beautiful Trees
Conceived and Developed By :- Developing and Marketing partner ABS Channel Partner ABS Promoters & Developers Head Office :- 09A-2, Dilshad Garden, Delhi-110092 Pacific Constructions & Management Futuresecure Invests Farm Site Office :- 30 KM Milestone, Dehradun, Saharapur Road, D-64, Ist Floor, Vikas Marg, Shakarpur, Delhi-110092 Village Ganeshpur, Distt Saharanpur A private heaven Imaging living in a farm surrounded by a garden full of colourful flowers and beautiful trees. Imagine staring at calm long stretches of nothingness. Wilderness of the jungle Imagine waking up to the sweet chirping of birds every morning. Classical yet contemporary It" be a place you"ll fall in love... Nature knocking on your front door Farms at Sukoon will be an awesome experience with wide variety of lifestyle. Facilities to suit your lifestyle Discover Yourself ?Hi-tech security arrangements. ?Esthetically and lavishly furnished cottage ? ?Beautiful landscaped garden Security guards. ? ?Fully secure boundary wall with entrance gate 24 hour water supply. ?Barbeque place ?100 % power backup for common facilities. ?Bonfire place ?Fully equiped beautifully landscaped club with ?Open area 90% and construction - 10% (Maximum) swimming pool. ?Serene surroundings admist wilderness of jungle ?In house grocery shop to meet your daily needs. ?In house maintenance services. Magical by Night Beautiful by Day Site map with local distances Mussoorie Kanatal Paonta Sahib Dhanaulti 19 Dehradun Sukoon – Dehradun Kms Mohand Rishikesh Rajaji National 47 Farm park Sukoon – Mussoorie Kms Haridwar Roorkee 71 Sukoon – Haridwar Kms 61 Sukoon – Rishikesh Kms Muzaffarnagar 71 Sukoon – Dhanaulti Kms 95 I am at peace Sukoon – Kanatal Kms 63 I am at rest Meerut Sukoon – Paonta Sahib Kms Modinagar I am home.... -
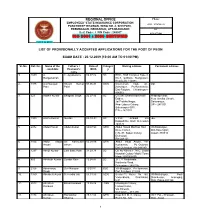
List of Provisionally Accepted Applications for the Post of Peon
REGIONAL OFFICE Phone: EMPLOYEES’ STATE INSURANCE CORPORATION 0135 – 2774762 / 63 PANCHDEEP BHAWAN, WING NO. 4, SHIVPURI, PREMNAGAR, DEHRADUN, UTTARAKHAND Fax Beat Code : I PIN Code : 248007 0135-2771542 ISO 9001 : 2000 CERTIFIED CHINTA SE MUKTI LIST OF PROVISIONALLY ACCEPTED APPLICATIONS FOR THE POST OF PEON EXAM DATE : 20.12.2009 (10:00 AM TO 01:00 PM) Sl. No. Ref. No. Name of the Father’s / Date of Categor Mailing address Permanent address candidate Husband’s Birth y name 1. 1859 A. C. Ayyaswamy 06.07.82 SC ESIC, Staff Complex Type-3, Ranganathan No-7, O-Block, Mangolpuri, New Delhi-110083 2. 1375 A.D.Narayan Shyam Kumar 01.06.83 GEN. C/o-Hetram Naik, AT- DO Patel Patel jhanakpur, Po-Baramkela, Dist-Raigarh, Chhattisgarh- 496551 3. 323 Aadhin Kumar Bhagwat Singh 20.07.86 SC C/o Shri Dharmendra Nath Pradeep Vihar, Dubey, Near Gavlira Chowk, Jai Prabha Nagar, Saharanpur, Near Labour Colony, UP – 247 001 Saharanpur (UP), PIN – 247 001 4. 1995 Aashu Kumar Gurdas 08-10-84 SC V.P.O. Ambadi Via -do- Dakpaththa Distt Dehradun 248125 5. 2072 Abdul Hamid Abdul shukur 08/07/90 GEN Abdul Hamid Marfhan Havi Vill-Barkanpur, Deve Camuli, Dist-Karemganj H.N.-97, Saken Colony, Assam-788810 Dehradun Pin-248121 6. 1104 Abdul Majid Sri Kalimullah 05.08.89 GEN Abdul Majid Ansari, Vill- Ansari Ansari Kurkawala, Po.-Doiwala, Dist-Dehradun, Pin-248140 7. 1297 Abhijit Kumar Late Babu Ram 31.03.78 SC Qtr.No-Reena F 7N.C, Jindal Hospital Colony Model Town Hisar-125005(H.R) 8. -

Kanatal - Rishikesh
DELHI - KANATAL - RISHIKESH Distance to Kanatal : 325 kms. (7 to 8 hrs drive) - Route : Delhi - Meerut - Muzaffarnagar - Roorkee - Haridwar - Rishikesh - Narendra Nagar - Chamba - Kanatal (On Chamba - Mussorie Road) USP of Kanatal - At 8500 ft above sea level, secluded, Awesome view of Himalayas. Clear view of Trishul, Chaukhamba and Bandarpoonch. Lots of trekking options in Kodia Jungle. Pine and Oak forest. Places to See - Kodia Jungle, Tehri Dam, Dhanolti - Surkanda devi temple, ECO Park. Road Condition - Road after Muzaffarnagar to Roorkee is bad. High traffic in Haridwar and Roorkee. Midway Break - Namaste Midway has MCD, Haldiram, Costa, Baskin and Robins is about 5 kms before Muzaffarnagar and an ideal stop over. Of course Cheetal Grand is also another option which is 12 km before Midway. Stay - 3 nights at Kanatal Club Mahindra. 1 night stay at Rishikesh GMVN Rishilok Tourist Rest House. The Journey We started by 5.30 Am. By 7.30 am we reached MCD. 10 am we were in Haridwar and by 1 pm reached Kanatal. Day - 1 Check in was smooth and post lunch we lazed around for the day... The resort is on the main Chamba - Dhanaulti road. Medium sized resort with about 35 rooms. All rooms are valley facing. Well appointed rooms with good insulation and room heating . Evenings and early morning are cold and some times windy too. The hotel is just infront of Kodia eco park. Food is pricey but members have discount and fun dining option. Monkey menace a problem. Day-2 We planned to trek Kodia Jungle. Morning trek was about 7 kms. -

Rishikesh and Kanatal Tour 2019
THRILLING - RISHIKESH & KANATAL with ROBBERS CAVE 09 Nights/10 Days 24th March – 02nd April 2019 Specially Designed for Indian High School… HIGHLIGHTS OF THE TOUR • Campfire • Watersports and Tehri lake • River Raing • Neergarh WaterFall • Community Interac5on • Flying fox • Rock Climb • WildliFe Safari at Chilla Naonal • Rapelling Park • Kayaking • Forest Research Ins5tute • Team Building Games, • White water raing Australian trolley, treasure hunt, lemon spoon, music chair • Dhanaul5 • Trek to Surkanda Devi • Robbers Cave FLIGHT DETAILS Depar5ng on 23rd Mar by 9W 547 From Dubai at 1100pm, reaching Delhi by 0355am on 24rd Mar Depar5ng on 24th Mar by 9W 633 From Delhi at 0600am, reaching Dehradun by 0655am on 24th Mar Depar5ng on 02nd Apr by 9W 639 From Dehradun at 1230pm, reaching Delhi by 0125pm on 02nd Apr Depar5ng on 02nd Apr by 9W 588 From Delhi at 0405pm, reaching Dubai by 0610pm on 02nd Apr DAY 1 : ARRIVAL IN KANATAL • Arrival at Dehradun airport and transFer to Kanatal (116 Kms – Drive 4 hours) • Check in to Camp • Lunch at Camp • Later Camp ac5vi5es • Camp fire • Dinner and overnight stay at Campsite. DAY 2 : KANATAL • After breakfast, • Full day for activities like rock climb, rappelling, valley crossing and lying fox. • Evening camp 9ire, snacks, dinner and night stay at camp. DAY 3 : KANATAL • After breakfast, Trek to Surkanda devi back to camp for lunch • Drive to Tehri lake • Enjoy water sports like speed boating, banana boat ride • Back to camp by evening • Camp 9ire and Overnight at Campsite DAY 4 : KANATAL - RISHIKESH • A,er breakFast, Drive to Rishikesh (80 Km’s – 3 hours) • Check into camp • Lunch at Camp • Camp games - Volley Ball, Badminton, Cricket, Tree climb • Snacks • Camp fire • Dinner and overnight in Campsite. -
Brochure Kanatal Wildex
Camp Wildex, Kanatal Mussoorie - Chamba Road, Uttarakhand CAMP WILDEX, KANATAL is situated at 8,400 fts near Mussoorie tucked away in the middle of the Kaudia Forest. Set in the heart of the Indian Himalayas and surrounded by dense, long wooden slopes of Deodar, Oak and Pine forests, Kanatal (Thangdhar) the camp offers tranquility seems to be straight out of some romantic classic. This is the chilliest offbeat destination near Delhi / NCR. The beautiful and picturesque 180 degree view of the snow clad Himalayas will fill you with a romantic charm. Beautiful and captivating environment and camp life will make you feel the love and pleasure of lying in Mother Nature's lap. BASIC FACTS: SEASON: Throughout the year TEMPERATURE: Varies from 4°c to 25°c (woolens are required) DISTANCE FROM MAJOR PLACES: Delhi: 300 kms | Chandigarh: 275 kms | Rishikesh: 75 kms Dehradun: 75 kms | Mussoorie: 42 kms | Chamba: 14 kms | Dhanaulti: 15 kms CAMP FACILITIES: . Stay in Swiss Camps / Rooms / Safari Camps . Buffet meals as per fixed menu . Electricity connection with power backup . Activity Hall for indoor Games/Group activity . Conference Area with Audio/Video facility ACTIVITIES POSSIBLE FROM THE CAMP: . Daily Bonfire with light Music . Rope Activities like Rock climbing, Rappelling & Valley crossing . Indoor games: Carom, Chess, Cards, Tambola etc. Obstacle courses like Burma Bridge, Parallel rope, etc. Team Building Activities / Group Games . Trek to Kaudia Forest / Surkanda Devi Temple (10,000 fts) NEARBY PLACES TO VISIT: Surkanda Devi Temple 7 kms | Dhanaulti Eco Park 13 kms | Tehri Dam 30 kms | Mussoorie 42 kms ACCOMMODATION: We have Swiss Cottage Camps of 15fts x 15fts in size, Rooms of 12fts x 12fts and Safari Camps of 10fts x 10fts. -
299161603.Pdf
YEAR YEA BATC SR NAME NICK DOB H FROM R TO H ADDRESS ADDRESS1 CITY COUNTRY PHONE PHONE1 MOBILE E-MAIL 1 A. BENERJEE C/O P. BENERJEE TRACTOR LTD. NO. 1, TARATOLLA RODA, GARDEN RD KOLKATA INDIA 2 A. DAVID ANGAMI 1996 1996 WAR CEMETERY KOHIMA NAGALAND INDIA 3 A. J. GEORGE BRO. PRINCIPAL, ST. JOSEPH ACADMEY DEHRADUN INDIA 0135-2712939 0135-2712071 9458131266 [email protected] 4 A. K. JOSHI 1947 1953 1953 NOIDA INDIA 0120-25452053 5 A. M. THANICKEN FSP BRO. 1960 1962 1962 ST. PATRICK, JUNIORATE ADYAAR CHENNAI - 600020 INDIA 6 A. P. S. CHAUHAN PP. SUNITA CHAUHAN DELHI INDIA 0120-6515560 011-26352801 7 A. P. SINGH 1965 1966 1966 MUSSOORIE INDIA 8 A. RAWLINS TEACHER 148, BURDWOOD HIGHWAY, BURDWOOD EAST VICTORIA-3151 AUSTRALIA 9 A. S. BEDI BRIG. TONY 284, SECTOR 11 - A CHANDIGARH INDIA 0172-2747762 10 A. S. PANWAR DR. INDIA 11 A. S. SANDHU LT.GEN. PVSM 15.2.40 1948 1955 1955 F-605 , SOM VIHAAR R.K. PURAM NEW DELHI - 110022 INDIA 0172-2606959 9810447730 12 AAKASH DEEP 2.5.91 C 2001 2009 2009 T/501, BLOCK - B, OM NIRMALAYA APPTS NAGESHWAR COLONY BOUINGR, PATNA 13 ABDULLAH MOHD 1979 ZAKARIA HOUSE, E-37, KALINDI COLONY, NEW DELHI INDIA 9810188034 14 ABDUS SAMAD HASHMI 1998 1998 TAHA SABBAN ESTATE P.O. BOX 9661 JEDDAH SAUDI ARABIA 15 ABEN LAL CHOTA MANGO 29.05.54 M 1963 1969 1969 FLAT NO.- C-8/8292 VASANT KUNJ, NEW DELHI INDIA 011-25672261 011-26133599 9810997722 [email protected] 16 ABHAY JIT JOSHI 1.12.92 C 2001 2009 2009 15/ 13, SUBHASH NAGAR HALDWANI UTRANCHAL INDIA 05942-237227 9412968275 [email protected] 17 ABHAY KUMAR M 1994 2002 2002 OPP, MANGLAM ENCLAVE MORE PO. -

Essence of Uttarakhand 06 Nights / 07 Days
Essence of Uttarakhand 06 Nights / 07 Days Package Highlights Haridwar: 01 Night • Har ki Pauri • Mansa Devi Temple Rishikesh: 02 Nights • Ram Jhula • Laxman Jhula • Shri Bharat Mandir Mussoorie: 03 Nights • Lal Tibba • Gun Hill • Kempty Waterfalls ***Entry Fees & Activity Cost to Be Paid Directly Wherever Applicable Meal: 06 Breakfast & 06 Dinner Hotels Used OR Similar: Destination Hotels & Resort Haridwar The Great Ananda or Similar Rishikesh Hotel Bliss Ganga or Regenta Inn On The Ganges or Similar Mussoorie Hotel Basera or Similar Package Cost: Package Cost Per Person Rates Per Adult Rate 23,500 /- CWB 19,000 /- CNB below 12 15,200 /- 5% GST on Total Tour Cost Suggested Itinerary Day 01: Delhi To Haridwar (Driving Hour: Delhi To Haridwar // 05 Hours. 15 min // 221 km) Arrive at Delhi Airport And you will be Transfer To Haridwar. Later, Check Into & Dinner at Hotel. Overnight, Stay at Hotel. Meal: Dinner Day 02: Haridwar To Rishikesh (Driving Hour: Haridwar To Rishikesh // Approx. 42 Min // 21.1 Km) After Breakfast check-out from hotel, Visit Mata Mansa Devi Temple - Is Very Popular Temple In Haridwar. This Temple Is Dedicated To Goddess Mata Mansa Devi. It Is Located At Bilwa Parvat ., Evening Visit Har Ki Pauri For Ganga Aarti. Har Ki Pauri Darshan Is Famous Across The Country Calling In Devotees To Attend The Darshan. Ultimately Making It The Center Of Attraction In Haridwar. The Literal Meaning Of The Name Har Ki Pauri Is ‘Har’ Meaning ‘Lord Shiva’, ‘Ki’ Meaning ‘Of’ And ‘Pauri’ Meaning ‘Steps.’ An Interesting Fact As To Why Har Ki Pauri Is Famous And Considered An Ideal Place For Worship Is Because It Is The Point Where The Ganga River Makes An Exit From The Mountain Terrains And Enters Into The Plains. -

Development of Adventure Tourism in Northern India : a Case Study of Uttrakhand
/ \ \ ✓ us rz 7G DEVELOPMENT OF ADVENTURE TOURISM IN NORTHERN INDIA : A CASE STUDY OF UTTRAKHAND THESIS SUBMITTED FOR THE AWARD OF THE DEGREE OF doctor of Vbilo0pbp IN COMMERCE (TOURISM) SAADIA LODI t rl Under the Supervision of Dr. SHEEBA HAMID (Associate Professor) [7T7?. DEPARTMENT OF COMMERCE ALIGARH MUSLIM UNIVERSITY ALIGARH (INDIA) 2013 14 NCV 2?14 k 1I1I 1 IU 1111k k\I1k Il III Department of Commerce on sheeba Hamid Aligarh Muslim University, .17A, (ph. (D. Aligarh. Associate (Professor (Tourism)'~'~-~' } Internal: 3505, 3506 Training a cPCPlaccement Officer External: 0571-2703661 Master of Tourism Administration (MTA) Qtertifttate This is to certify that the work embodied, in this thesis entitled "Development of Adventure Tourism in Northern India: A Case Study of (Jltrakhand" is the original work to the best of my knowledge. It has been carried out by Ms. Saadia Lodi, under my supervision and is suitable for submission for the award of Ph.D degree in Commerce (Tourism) of Aligarh Muslim University, Aligarh. br. Shee a Hamid (Associat4 Professor) 'Mussoorie Cottage', 4/1286-A, Sir Syed Nagar, Aligarh — 202002 Res: +91-571-2407861, Cell: +91-9319926119 E-mail: [email protected] LIST OF CONTENTS Page No. Preface i-iv Acknowledgements v-vii List of Tables viii-x List of Figures xi List of Abbreviations xii-xiii Chapter-1: Introductory Background and Framework 1-35 of the Study (Section: A) 1. Introductory Background 1-2 2. India: A Background vis-a-vis Tourism 2-3 3. What is Adventure Tourism? 3-4 4. Uttarakhand: Adventure Tourism Destination 4 (Section: B) 5.