Abbvie (Solvay Pharmaceuticals) 00032
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2009: Turning the Corner
data page 2009: Turning the corner Walter Yang Despite the shaky start to 2009, the biotech sector regained its financial Although initial public offerings showed signs of resuscitation (at least footing. Biotech indices were up, as were offerings and partnership mon- 13 more companies are now in the queue), follow-on financings came in ies. Excluding collaborations, the sector raised a total of $24.3 billion. above $6 billion—the second-best year over the past decade. Stock market performance Global biotech industry financing Although biotech indices were up ~16% last year, they underperformed The boost in partnership promises to US biotechs and follow-on financings other major indices. pushed industry funding to $61.3 billion, up 82% from 2008. 1,500 Swiss Market S&P 500 2009 36.9 10.0 5.1 2.2 6.0 0.9 1,400 NASDAQ Biotech Dow Jones 2008 20.0 3.2 5.3 3.1 1.9 0.1 1,300 NASDAQ BioCentury 100 2007 22.4 11.7 6.8 4.7 4.4 3.0 1,200 Partnering 2006 19.8 11.9 5.6 4.7 5.6 2.0 1,100 Year Debt and other Index 17.3 6.1 5.4 2.7 4.8 1.9 1,000 2005 Venture capital PIPEs 900 2004 10.9 8.8 5.3 2.9 3.3 2.6 Follow-ons 800 2003 8.9 9.1 4.0 2.2 3.9 0.5 IPOs 700 010203040506070 Amount raised ($ billions) 1/09 2/09 3/09 4/09 5/09 6/09 7/09 8/09 9/09 12/08 Month ending 10/09 11/09 12/09 Partnership figures are for deals involving a US company. -

ALEXION PHARMACEUTICALS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K Current Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of earliest event reported): May 4, 2021 ALEXION PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) Delaware 000-27756 13-3648318 (State or other jurisdiction of incorporation) (Commission File Number) (IRS Employer Identification No.) 121 Seaport Boulevard, Boston, Massachusetts 02210 (Address of principal executive offices, including zip code) (475) 230-2596 (Registrant’s telephone number, including area code) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: ☒ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) ☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) ☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) ☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Securities registered pursuant to Section 12(b) of the Act: Trading Name of each exchange Title of each class Symbol on which registered Common Stock, par value $0.0001 per share ALXN The Nasdaq Global Select Market Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). -

Overview of Ftc Antitrust Actions in Pharmaceutical Services and Products
OVERVIEW OF FTC ANTITRUST ACTIONS IN PHARMACEUTICAL SERVICES AND PRODUCTS Health Care Division Bureau of Competition Federal Trade Commission Washington D.C. 20580 Markus H. Meier Assistant Director Bradley S. Albert Deputy Assistant Director Saralisa C. Brau Deputy Assistant Director September 2009 TABLE OF CONTENTS Page I. INTRODUCTION. ........................................................... 1 II. CONDUCT INVOLVING PHARMACEUTICAL SERVICES AND PRODUCTS. 3 A. Monopolization. ...................................................... 3 B. Agreements Not to Compete. ............................................ 8 C. Agreements on Price or Price-Related Terms. 14 D. Agreements to Obstruct Innovative Forms of Health Care Delivery or Financing. 20 E. Illegal Tying and Other Arrangements. .................................... 20 III. PHARMACEUTICAL MERGERS. ........................................... 20 A. Horizontal Mergers Between Direct Competitors. 20 B. Potential Competition Mergers. ......................................... 44 C. Innovation Market Mergers. ............................................ 47 D. Vertical Mergers...................................................... 49 IV. INDUSTRY GUIDANCE STATEMENTS...................................... 50 A. Advisory Opinions. ................................................... 50 B. Citizen Petition to the Food and Drug Administration. 51 V. AMICUS BRIEFS. ......................................................... 51 VI. INDICES. ............................................................ -

1976 3M Medical Solutions Division 2019 Electrocore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc
CURRENT SUSTAINING MEMBER COMPANIES MEMBER FOR OVER: 10 Years 25 Years 50 Years Member Since (alphabetical order) 1976 3M Medical Solutions Division 2019 electroCore, Inc. 2019 Optinose 1985 Abbott Laboratories, Inc. 2010 Endo Pharmaceuticals 2018 Organogenesis 2013 AbbVie Inc. 2017 Exelixis 2004 Otsuka America Pharmaceutical, Inc. 2021 Adaptive Biotechnologies 2016 Express Scripts Federal Pharmacy 2018 Pacira BioSciences, Inc. 2017 ACADIA Pharmaceuticals, Inc. 2010 Federal Practitioner 2018 Paratek Pharmaceuticals 2020 AcelRx Pharmaceuticals, Inc. 2018 Foundation Medicine, Inc. 1990 Pfizer Pharmaceuticals 2020 Acorda Therapeutics 2021 Frontier Technology Inc. (FTI) 2017 Pharmacyclics, LLC 2019 Aimmune 2020 Fresenius Medical Care North America 2020 RedHill BioPharma 2003 Alcon Laboratories, Inc. 1989 Genentech Inc. 2019 Red One Medical 2019 Alexion Pharmaceuticals, Inc. 2006 Gilead Sciences 2020 Regeneron 2017 Alkermes, Inc. 1983 GLAXOSMITHKLINE 2009 Regenesis Biomedical, Inc. 2019 Alnylam Pharmaceuticals 2013 Golden State Medical Supply, Inc. 2011 Remund Group, LLC 2019 Altarum Institute 2020 GRAIL 2018 Rigel Pharmaceuticals 2020 Amarin Corporation 2019 Greenwich Biosciences 2000 Sanofi 1994 AmerisourceBergen 2013 Gulf Coast Pharmaceuticals Plus, LLC 2020 Seattle Genetics 1992 Amgen 2008 Heritage Health Solutions, Inc. 2004 Siemens Medical Solutions 2020 Amneal Pharmaceutical 2017 Hill-Rom Company 2019 SK Life Science, Inc. 2019 Aptive Resources LLC 2020 Immunomedics 2002 Smith & Nephew, Inc. 2020 The Arbinger Institute 2019 ImmunoVation, LLC 2019 Sobi Inc. 2011 Arbor Pharmaceuticals, LLC 2019 Incyte Corporation 2013 Stryker Orthopaedics 2010 Argentum Medical, LLC 2019 Indivior 2018 Sun Pharmaceutical 2019 ASM Research, LLC 2015 Intercept Pharmaceuticals 1999 Sunovion Pharmaceuticals, Inc. 1986 Astellas Pharma US, Inc. 2019 Ipsen Biopharmaceuticals, Inc. 2016 Taiho Oncology, Inc. 1995 AstraZeneca 2018 IT Cadre 2015 Takeda Oncology 2020 Baudax Bio, Inc. -

Pharma: Strategic Realignment for a 112/113 Better Future Prism / 2 / 2020
Pharma: Strategic realignment for a 112/113 better future Prism / 2 / 2020 Pharma: Strategic realignment for a better future ... and how the industry will be forced to overcome its hesitation to innovate in operations Ben van der Schaaf, Aurelien Guichard The life sciences sector faces significant impact from Amid the search for COVID-19 – and although the race for treatments and vaccines effective COVID-19 treatments and vaccines, is dominating the headlines, the effect on the industry will the pandemic will have not solely be positive. long-term side effects for the global pharmaceutical In the short term, some companies industry. As our article explains, companies are laying people off and reducing will need to focus on operations, whereas others are change in three areas reallocating resources to focus on (portfolio reprioritization, COVID-19, or even ramping up accelerated R&D and technology efforts in other areas. Stock-market transformation) if they performance has been as diverse are to position (Figure 1). themselves successfully for the future. Organizations need to consider major strategic questions now, to ensure their success in the longer term. The industry is clearly in the middle of the efforts to combat COVID-19: - More than 20 companies are trying to find a treatment with either new or approved drugs1. - More than 15 companies globally are mobilizing resources to develop new vaccines2. - Globally, by the end of May, more than 1,300 clinical trials related to COVID-19 were recruiting patients3. 1. Marketwatch.com, 6 May 2020 2. Drugtargetreview.com, 9 April 2020 3. clintrials.gov, 31 May 2020 Pharma: Strategic realignment for a 114/115 better future Prism / 2 / 2020 2. -

28 May 2009 Thomas E. Costa
The Third International Pharmaceutical Regulatory and Compliance Congress and Best Practices Forum Thomas E. Costa 2828 MayMay 20092009 This presentation represents my own personal opinion and is not the official position of Bristol-Myers Squibb PhRMA Code 2009 • Updated in response to concerns of healthcare stakeholders • Reaffirms that interactions between pharmaceutical company representatives and healthcare professionals (HCPs) should: ¾ Inform HCPs about the benefits and risks of our products ¾ Provide scientific and educational information ¾ Obtain feedback and advice about our products through consultation with medical experts • The PhRMA Code is the new industry standard PhRMA Code Signatory Companies • Abbott • Eli Lilly and Company • Amgen, Inc. • Merck & Company, Inc. • Amylin Pharmaceuticals, Inc. • Millennium Pharmaceuticals, Inc. • Astellas US LLC • Novartis Pharmaceuticals • AstraZeneca LP Corporation • Bayer HealthCare • Novo Nordisk Inc. Pharmaceuticals • Otsuka America, Inc. • Boehringer Ingleheim • Ovation Pharmaceuticals, Inc. Pharmaceuticals, Inc. • Pfizer, Inc. • Bristol-Myers Squibb Company • Purdue Pharma LP • Cephalon, Inc. • sanofi-aventis US • Covidien Ltd. • Schering-Plough Corporation • Daiichi Sankyo, Inc. • Sepracor, Inc. • Eisai, Inc. • Signa-Tau Pharmaceuticals, Inc. • EMD Serono • Solstice Neurosciences, Inc. • Endo Pharmaceuticals, Inc. • Solvay Pharmaceuticals, Inc. • Genzyme Corporation • Takeda Pharmaceuticals North • GlaxoSmithKline America, Inc. • Hoffmann-La Roche, Inc. • Wyeth • Johnson and Johnson -

References Used in Algorithms for the Treatment of Persons with Crohn’S Disease
REFERENCES USED IN ALGORITHMS FOR THE TREATMENT OF PERSONS WITH CROHN’S DISEASE 1. AA Pharma Inc: Winpred (prednisone). In: CA Product Monograph. Vaughan, ON; 2018. 2. AbbVie Corporation: Humira (adalimumab). In: CA Product Monograph. St Laurent, QC; 2019. 3. AbbVie Inc: Humira (adalimumab). In: US Product Monograph. North Chicago, IL; 2020. 4. Amgen Canada Inc: Avsola (infliximab). In: CA Product Monograph. Mississauga, ON; 2020. 5. Amgen Inc: Amjevita (adalimumab-atto). In: US Product Monograph. Thousand Oaks, CA; 2019. 6. Amgen Inc: Avsola (infliximab-axxq). In: US Product Monograph. Thousand Oaks, CA; 2019. 7. Antares Pharma Inc: Methotrexate. In: FDA Product Monograph. Ewing, NJ; 2019. 8. Apotex Inc: Methotrexate. In: CA Product Monograph. Toronto, ON; 2019. 9. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A: NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. Journal of gastroenterology 2016, 51(1):22-29. 10. Aspen Pharmacare Canada Inc: Imuran (azathioprine). In: CA Product Monograph. Oakville, ON; 2019. 11. Biogen Canada Inc: Tysabri (natalizumab). In: CA Product Monograph. Mississauga, ON; 2017. 12. Biogen Idec Inc: Tysabri (natalizumab). In: US Product Monograph. Cambridge, MA; 2019. 13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics 2015, 98(1):19-24. 14. Boehringer Ingelheim Pharmaceuticals Inc: Cyltezo (adalimumab-adbm). In: US Product Monograph. Ridgefield, CT; 2019. -

ASN Kidney Week 2020 Reimagined: Disclosures Page 1
10/14/2020 ASN Kidney Week 2020 Reimagined: Disclosures Page 1 Last Name First Name Nothing Employer Consultancy Ownership Interest Research Funding Honoraria Patents or Inventions Scientific Advisor or Membership Speakers Bureau Other Interests or Relationships to Disclose Abdel-Kader Khaled Vanderbilt University Medical Center BMC Nephrology; CJASN NKF Education committee; NIDDK Health IT work group Abudayyeh Ala University of Texas MD Anderson Cancer Center Adler Sharon Retrophin; Bristol Myers Squibb; Bayer; Retrophin; Bayer; ChemoCentryx; Omeros; Zyversa Bayer; Zyversa; Retrophin; AstraZeneca; Morphosys Retrophin; Bayer Pharmaceuticals; Zyversa; KDIGO; KRN; NephCure Kidney International AstraZeneca; ChemoCentryx; Omeros; Zyversa; Therapeutics; Calliditas; Morphosys AstraZeneca; Morphosys Foundation; Karger Publishers Morphosys; Karger Afkarian Maryam University of California, Davis Afrouzian Marjan University of Texas Medical Branch Alexion Pharmaceuticals; Banff Foundation Afshinnia Farsad Agarwal Anupam University of Alabama at Birmingham Dynamed - review content related to AKI for Goldilocks Therapeutics Genzyme/Sanofi Fabry Fellowship Award Univ Southern California, Vanderbilt, Emory, Akebia Editorial Board of AJP Renal, Kidney Int and My wife, Lisa Curtis, will be President-elect Dynamed and review updated materials prepared by Lab Investigation; invited to serve on for Women in Nephrology (2018-2019). Dynamed editorial team for AKI topics. Akebia - Advisory board of Goldilocks Therapeutics, Expert Panel to review new therapeutics -

Bio/Pharmaceutical Outsourcing Report: November 2016
Volume 21, Number 11, November 2016 Bio/Pharmaceutical Outsourcing Report Part of PharmSource STRATEGIC ADVANTAGE Business Conditions 1 Business Conditions 1 PE-Owned Companies in Line to Change Hands PE-Owned Companies in Line to Change Hands 2 Commercial Dose Manufacturing and Mergers and acquisitions are a hot topic in the contract services industry these Packaging days, and there is a lot of curiosity about which companies are likely to change 2 New Manufacturing Deal, Temporary hands. Of course, any company is a target at any time, especially when the price is Closures and Recall for Patheon right, but insight to likely acquisition candidates can be gained by looking at the list 3 Commercial Dose Manufacturing and Packaging in Brief 3 Clinical Dose Manufacturing and leastof companies four years owned ago are by likelyprivate targets equity in (PE) the nextfirms. year On oraverage, two. PE firms tend to hold Packaging onto their investments for about five years, so companies that were acquired at 3 Marken to be Acquired by UPS The table on page 2 lists 23 CMC services companies that are PE-owned; the list 5 Clinical Dose Manufacturing and was derived from the PharmSource Strategic Advantage database “Search by Packaging in Brief Ownership” feature. It is sorted by the year each company was acquired, and 6 Side Effects: Impacts of Key Events on suggests that at least 13 CMOs and CDMOs are ripe for a transaction in the foresee- CMOs and CROs able future. 7 API — Large Molecule The sale of one company, Marken (Durham, N.C., USA), was announced just this 7 Samsung BioLogics Completes IPO month (see news item, Page 3); another, Capsugel (Morristown, N.J., USA), has been 8 API — Large Molecule in Brief mentioned in the business press as being prepared for an ownership change. -

Comparison of Oral Contraceptives and Non-Oral Alternatives
PL Detail-Document #290305 −This PL Detail-Document gives subscribers additional insight related to the Recommendations published in− PHARMACIST’S LETTER / PRESCRIBER’S LETTER March 2013 Comparison of Oral Contraceptives and Non-Oral Alternatives —More information about the use of contraceptives is available in our PL Detail-Document, Hormonal Contraception— Productsa Manufacturerb Estrogen Progestin LOW-DOSE MONOPHASIC PILLS Aviane-28 Teva EE 20 mcg Levonorgestrel 0.1 mg Falmina Novast Lessina Teva Lutera Actavis Orsythia Qualitest Sronyx Actavis Gildess Fe 1/20 Qualitest EE 20 mcg Norethindrone acetate Junel 1/20 Teva 1 mg Junel Fe 1/20 Teva Loestrin-21 1/20 Warner Chilcott/Teva Loestrin Fe 1/20 Warner Chilcott/Teva Microgestin 1/20 Actavis Microgestin Fe 1/20 Actavis Generess Fe chewable Actavis EE 25 mcg Norethindrone 0.8 mg Altavera Sandoz EE 30 mcg Levonorgestrel 0.15 mg Kurvelo Lupin Levora Actavis Marlissa Glenmark Nordette-28 Duramed/Teva Portia-28 Teva Cryselle-28 Teva EE 30 mcg Norgestrel 0.3 mg Elinest Novast Low-Ogestrel-21 Actavis Low-Ogestrel-28 Actavis Lo/Ovral-28 Wyeth Gildess Fe 1.5/30 Qualitest EE 30 mcg Norethindrone acetate Junel 1.5/30 Teva 1.5 mg Junel Fe 1.5/30 Teva Loestrin 1.5/30-21 Warner Chilcott/Teva Loestrin Fe 1.5/30 Warner Chilcott/Teva Microgestin 1.5/30 Actavis Microgestin Fe 1.5/30 Actavis More. Copyright © 2013 by Therapeutic Research Center 3120 W. March Lane, Stockton, CA 95219 ~ Phone: 209-472-2240 ~ Fax: 209-472-2249 www.PharmacistsLetter.com ~ www.PrescribersLetter.com ~ www.PharmacyTechniciansLetter.com -
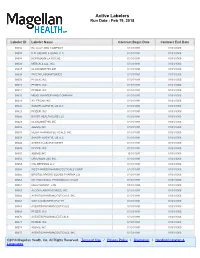
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Why Are Some Generic Drugs Skyrocketing in Price? Hearing Committee on Health, Education, Labor, and Pensions United States Sena
S. HRG. 113–859 WHY ARE SOME GENERIC DRUGS SKYROCKETING IN PRICE? HEARING BEFORE THE SUBCOMMITTEE ON PRIMARY HEALTH AND AGING OF THE COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS UNITED STATES SENATE ONE HUNDRED THIRTEENTH CONGRESS SECOND SESSION ON EXAMINING THE PRICING OF GENERIC DRUGS NOVEMBER 20, 2014 Printed for the use of the Committee on Health, Education, Labor, and Pensions ( Available via the World Wide Web: http://www.gpo.gov/fdsys/ U.S. GOVERNMENT PUBLISHING OFFICE 24–459 PDF WASHINGTON : 2017 For sale by the Superintendent of Documents, U.S. Government Publishing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Nov 24 2008 13:32 May 19, 2017 Jkt 000000 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 S:\DOCS\24459.TXT DENISE HELPN-003 with DISTILLER COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS TOM HARKIN, Iowa, Chairman BARBARA A. MIKULSKI, Maryland LAMAR ALEXANDER, Tennessee PATTY MURRAY, Washington MICHAEL B. ENZI, Wyoming BERNARD SANDERS (I), Vermont RICHARD BURR, North Carolina ROBERT P. CASEY, JR., Pennsylvania JOHNNY ISAKSON, Georgia KAY R. HAGAN, North Carolina RAND PAUL, Kentucky AL FRANKEN, Minnesota ORRIN G. HATCH, Utah MICHAEL F. BENNET, Colorado PAT ROBERTS, Kansas SHELDON WHITEHOUSE, Rhode Island LISA MURKOWSKI, Alaska TAMMY BALDWIN, Wisconsin MARK KIRK, Illinois CHRISTOPHER S. MURPHY, Connecticut TIM SCOTT, South Carolina ELIZABETH WARREN, Massachusetts DEREK MILLER, Staff Director LAUREN MCFERRAN, Deputy Staff Director and Chief Counsel DAVID P. CLEARY, Republican Staff Director SUBCOMMITTEE ON PRIMARY HEALTH AND AGING BERNARD SANDERS, Vermont, Chairman BARBARA A.