Estimating the Economic Costs of Antimicrobial Resistance: Model And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Life Members As on 20Th January 2021
LIST OF LIFE MEMBERS AS ON 20TH JANUARY 2021 10. Dr. SAURABH CHANDRA SAXENA(2154) ALIGARH S/O NAGESH CHANDRA SAXENA POST HARDNAGANJ 1. Dr. SAAD TAYYAB DIST ALIGARH 202 125 UP INTERDISCIPLINARY BIOTECHNOLOGY [email protected] UNIT, ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 11. Dr. SHAGUFTA MOIN (1261) [email protected] DEPT. OF BIOCHEMISTRY J. N. MEDICAL COLLEGE 2. Dr. HAMMAD AHMAD SHADAB G. G.(1454) ALIGARH MUSLIM UNIVERSITY 31 SECTOR OF GENETICS ALIGARH 202 002 DEPT. OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY 12. SHAIK NISAR ALI (3769) ALIGARH 202 002 DEPT. OF BIOCHEMISTRY FACULTY OF LIFE SCIENCE 3. Dr. INDU SAXENA (1838) ALIGARH MUSLIM UNIVERSITY, ALIGARH 202 002 HIG 30, ADA COLONY [email protected] AVANTEKA PHASE I RAMGHAT ROAD, ALIGARH 202 001 13. DR. MAHAMMAD REHAN AJMAL KHAN (4157) 4/570, Z-5, NOOR MANZIL COMPOUND 4. Dr. (MRS) KHUSHTAR ANWAR SALMAN(3332) DIDHPUR, CIVIL LINES DEPT. OF BIOCHEMISTRY ALIGARH UP 202 002 JAWAHARLAL NEHRU MEDICAL COLLEGE [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 14. DR. HINA YOUNUS (4281) [email protected] INTERDISCIPLINARY BIOTECHNOLOGY UNIT ALIGARH MUSLIM UNIVERSITY 5. Dr. MOHAMMAD TABISH (2226) ALIGARH U.P. 202 002 DEPT. OF BIOCHEMISTRY [email protected] FACULTY OF LIFE SCIENCES ALIGARH MUSLIM UNIVERSITY 15. DR. IMTIYAZ YOUSUF (4355) ALIGARH 202 002 DEPT OF CHEMISTRY, [email protected] ALIGARH MUSLIM UNIVERSITY, ALIGARH, UP 202002 6. Dr. MOHAMMAD AFZAL (1101) [email protected] DEPT. OF ZOOLOGY [email protected] ALIGARH MUSLIM UNIVERSITY ALIGARH 202 002 ALLAHABAD 7. Dr. RIAZ AHMAD(1754) SECTION OF GENETICS 16. -

Immunology and Cell Biology of Parasitic Diseases 2011
Journal of Biomedicine and Biotechnology Immunology and Cell Biology of Parasitic Diseases 2011 Guest Editors: Luis I. Terrazas, Abhay R. Satoskar, and Jorge Morales-Montor Immunology and Cell Biology of Parasitic Diseases 2011 Journal of Biomedicine and Biotechnology Immunology and Cell Biology of Parasitic Diseases 2011 Guest Editors: Luis I. Terrazas, Abhay R. Satoskar, and Jorge Morales-Montor Copyright © 2012 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Journal of Biomedicine and Biotechnology.” All articles are open access articles distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board The editorial board of the journal is organized into sections that correspond to the subject areas covered by the journal. Agricultural Biotechnology Ahmad Z. Abdullah, Malaysia Hari B. Krishnan, USA B. C. Saha, USA Guihua H. Bai, USA Carol A. Mallory-Smith, USA Abdurrahman Saydut, Turkey Christopher P. Chanway, Canada Xiaoling Miao, China Mariam B. Sticklen, USA Ravindra N. Chibbar, Canada Dennis P. Murr, Canada Kok Tat Tan, Malaysia Adriana S. Franca, Brazil Rodomiro Ortiz, Sweden Chiu-Chung Young, Taiwan Ian Godwin, Australia Encarnacion´ Ruiz, Spain Animal Biotechnology E. S. Chang, USA Tosso Leeb, Switzerland Lawrence B. Schook, USA Bhanu P. Chowdhary, USA James D. Murray, USA Mari A. Smits, The Netherlands Noelle E. Cockett, USA Anita M. Oberbauer, USA Leon Spicer, USA Peter Dovc, Slovenia Jorge A. Piedrahita, USA J. Verstegen, USA Scott C. Fahrenkrug, USA Daniel Pomp, USA Matthew B. Wheeler, USA Dorian J. Garrick, USA Kent M. -

News and Views Volume 17 No. 1 June 2018
Society for Scientific Values Ethics in Scientific Research Development and Management News and Views Volume 17 No. 1 June 2018 Editor Dr. Sanjeev Kumar Shrivastava Regd. Office: DST Centre for Visceral Mechanisms V. P. Chest Institute University of Delhi, Delhi 1 SSV News and Views 17 (1), June 2018 Executive Council Members President Treasurer Prof. K. L. Chopra Dr. Harikishan Ex-Director, IIT, Kharagpur Formerly Scientist, NPL, New Delhi. M-70, Kirti Nagar [email protected] New Delhi 110 015 [email protected], [email protected] Members: Dr. Vikram Kumar Phone: +91-11-25154114 Dr. Indra Nath Dr. Santa Chawla Vice Presidents Prof. B.V. Reddi Dr. Indramani Mishra Dr. J.C.Sharma Indian Agricultural Institute, Pusa, New Delhi Prof. Uttam Pati [email protected] Dr. Akhila Anand Phone: +91-11-25842294, 25071303 Dr. Rakesh Singh Prof. B.D. Malhotra Prof.Dean, N.School Raghuram, of Biotechnology, GGS Ms. Shubhrima Ghosh Indraprastha University, Sector 16C, Dwarka, New Delhi - 110078, Special Invitees INDIA Prof. P. N. Srivastava [email protected] Prof. Bimla Buti Dr. P. N. Tiwari Secretary Dr. R. K. Kotnala Website Scientist, NPL, New Delhi. Ms. Shubhrima Ghosh, IIT Delhi [email protected] Dr. Sanjeev Shrivastava, IISc Phone: +91-11-45608599, +91-11-45608266 Editor, Publications (News & Views) Joint Secretary Dr. Sanjeev Kumar Shrivastava Prof. S Major, (February 2017) IIT Mumbai CeNSE, IISc Bengaluru [email protected] Mob: 08277566371 ::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::::: -

Nominations for Padma Awards 2011
c Nominations fof'P AWARDs 2011 ADMA ~ . - - , ' ",::i Sl. Name';' Field State No ShriIshwarappa,GurapJla Angadi Art Karnataka " Art-'Cinema-Costume Smt. Bhanu Rajopadhye Atharya Maharashtra 2. Designing " Art - Hindustani 3. Dr; (Smt.).Prabha Atre Maharashtra , " Classical Vocal Music 4. Shri Bhikari.Charan Bal Art - Vocal Music 0, nssa·' 5. Shri SamikBandyopadhyay Art - Theatre West Bengal " 6: Ms. Uttara Baokar ',' Art - Theatre , Maharashtra , 7. Smt. UshaBarle Art Chhattisgarh 8. Smt. Dipali Barthakur Art " Assam Shri Jahnu Barua Art - Cinema Assam 9. , ' , 10. Shri Neel PawanBaruah Art Assam Art- Cinema Ii. Ms. Mubarak Begum Rajasthan i", Playback Singing , , , 12. ShriBenoy Krishen Behl Art- Photography Delhi " ,'C 13. Ms. Ritu Beri , Art FashionDesigner Delhi 14. Shri.Madhur Bhandarkar Art - Cinema Maharashtra Art - Classical Dancer IS. Smt. Mangala Bhatt Andhra Pradesh Kathak Art - Classical Dancer 16. ShriRaghav Raj Bhatt Andhra Pradesh Kathak : Art - Indian Folk I 17., Smt. Basanti Bisht Uttarakhand Music Art - Painting and 18. Shri Sobha Brahma Assam Sculpture , Art - Instrumental 19. ShriV.S..K. Chakrapani Delhi, , Music- Violin , PanditDevabrata Chaudhuri alias Debu ' Art - Instrumental 20. , Delhi Chaudhri ,Music - Sitar 21. Ms. Priyanka Chopra Art _Cinema' Maharashtra 22. Ms. Neelam Mansingh Chowdhry Art_ Theatre Chandigarh , ' ,I 23. Shri Jogen Chowdhury Art- Painting \VesfBengal 24.' Smt. Prafulla Dahanukar Art ~ Painting Maharashtra ' . 25. Ms. Yashodhara Dalmia Art - Art History Delhi Art - ChhauDance 26. Shri Makar Dhwaj Darogha Jharkhand Seraikella style 27. Shri Jatin Das Art - Painting Delhi, 28. Shri ManoharDas " Art Chhattisgarh ' 29. , ShriRamesh Deo Art -'Cinema ,Maharashtra Art 'C Hindustani 30. Dr. Ashwini Raja Bhide Deshpande Maharashtra " classical vocalist " , 31. ShriDeva Art - Music Tamil Nadu Art- Manipuri Dance 32. -

Adult Immunization
ADULT IMMUNIZATION Editors S.K. Sharma, R.K. Singal, A.K. Agarwal A Publication of the Association of Physicians of India For Private Circulation Only ADULT IMMUNIZATION (Monograph) Vol. 1, March 2009 Editors: Drs. S.K. Sharma, R.K. Singal, A.K. Agarwal © All Rights Reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. This book contains views and opinions of a group of experts and does not represent the decisions or stated policies of the Association of Physicians of India or the Editor(s). The publisher disclaims responsibility for opinions expressed by authors and contents of the manuscripts. Published by Dr. R.K. Singal Past-President, Association of Physicians of India For the Association of Physicians of India Unit No. 6 & 7, Turf Estate, Opp. Shakti Mills Compound Off Dr. E. Moses Road, Mumbai - 400 011 ADULT IMMUNIZATION Editors S.K. Sharma R.K. Singal A.K. Agarwal New Delhi New Delhi New Delhi Associate Editors Alladi Mohan Gautam Ahluwalia Tirupati Ludhiana Principal Editorial Advisors Y.P. Munjal S.K. Bichile New Delhi Mumbai Members – Editorial Board Sujit Kumar Bhattacharya Shashank R. Joshi Indian Council of Medical Research Mumbai Vinay Gulati O.P. Kalra New Delhi Delhi Pritam Gupta Sandhya Kamath New Delhi Mumbai D.G. Jain Jai P. Narain New Delhi World Health Organization Sanjay Jain S.C. Sharma Chandigarh New Delhi ADULT IMMUNIZATION Editors S.K. Sharma Chief, Division of Pulmonary, Critical Care and Sleep Medicine Professor and Head, Department of Medicine All India Institute of Medical Sciences Ansari Nagar, New Delhi - 110 029 R.K. -

Book Download
SOCIETY OF BIOLOGICAL CHEMISTS (INDIA) (1930 – 2011) 1 TABLE OF CONTENTS 1. Goals and activities of SBC(I) 2. Rules and Bye-laws of SBC(I) 3. Past Presidents, Secretaries, Treasurers (with tenure) 4. “Reminiscences on the development of the Society of Biological Chemists (India): a personal perspective” by Prof. N. Appaji Rao 5. “Growth of Biochemistry in India” by Prof. G. Padmanaban 6. Current office bearers 7. Current Executive Committee Members 8. Office staff 9. Past meeting venues of SBC(I) 10. SBC(I) awards, criteria and procedure for applying 11. SBC(I) awardees 12. Current list of life members with address 13. Acknowledgments 2 GOALS AND ACTIVITIES OF SBC(I) To meet a long felt need of scientists working in the discipline of biological chemistry " The Society Of Biological Chemists (India)" was founded in 1930, with its Head Quarters at Indian Institute of Science, Bangalore. It was registered under the Societies Act in the then princely state of Mysore and the memorandum of registration was signed by the late Profs. V. Subramanian, V. N. Patwardhan and C. V. Natarajan, who were leading personalities in the scientific firmament during that period. The Society played a crucial role during the Second World War by advising the Government on the utilization of indigenous biomaterials as food substitutes, drugs and tonics, on the industrial and agricultural waste utilization and on management of water resources. The other areas of vital interest to the Society in the early years were nutrition, proteins, enzymes, applied microbiology, preventive medicines and the development of high quality proteins from indigenous plant sources. -

Zone Wise List of NASI Fellows
The National Academy of Sciences, India (The Oldest Science Academy of India) Zone wise list of Fellows & Honorary Fellows (2021) 5, Lajpatrai Road, Prayagraj – 211002, UP, India 1 The list has been divided into six zones; and each zone is further having the list of scientists of Physical Sciences and Biological Sciences, separately. 2 The National Academy of Sciences, India 5, Lajpatrai Road, Prayagraj – 211002, UP, India Zone wise list of Fellows Zone 1 (Bihar, Jharkhand, Odisha, West Bengal, Meghalaya, Assam, Mizoram, Nagaland, Arunachal Pradesh, Tripura, Manipur and Sikkim) (Section A – Physical Sciences) ACHARYA, Damodar, Chairman, Advisory Board, SOA Deemed to be University, Khandagiri Squre, Bhubanesware - 751030; ACHARYYA, Subhrangsu Kanta, Emeritus Scientist (CSIR), 15, Dr. Sarat Banerjee Road, Kolkata - 700029; ADHIKARI, Satrajit, Sr. Professor of Theoretical Chemistry, School of Chemical Sciences, Indian Association for the Cultivation of Science, 2A & 2B Raja SC Mullick Road, Jadavpur, Kolkata - 700032; ADHIKARI, Sukumar Das, Formerly Professor I, HRI,Ald; Professor & Head, Department of Mathematics, Ramakrishna Mission Vivekananda University, Belur Math, Dist Howrah - 711202; BAISNAB, Abhoy Pada, Formerly Professor of Mathematics, Burdwan Univ.; K-3/6, Karunamayee Estate, Salt Lake, Sector II, Kolkata - 700091; BANDYOPADHYAY, Sanghamitra, Professor & Director, Indian Statistical Institute, 203, BT Road, Kolkata - 700108; BANERJEA, Debabrata, Formerly Sir Rashbehary Ghose Professor of Chemistry,CU; Flat A-4/6,Iswar Chandra Nibas 68/1, Bagmari Road, Kolkata - 700054; BANERJEE, Rabin, Emeritus Professor, SN Bose National Centre for Basic Sciences, Block - JD, Sector - III, Salt Lake, Kolkata - 700098; BANERJEE, Soumitro, Professor, Department of Physical Sciences, Indian Institute of Science Education & Research, Mohanpur Campus, WB 741246; BANERJI, Krishna Dulal, Formerly Professor & Head, Chemistry Department, Flat No.C-2,Ramoni Apartments, A/6, P.G. -
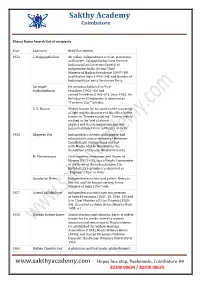
Sakthy Academy Coimbatore
Sakthy Academy Coimbatore Bharat Ratna Award: List of recipients Year Laureates Brief Description 1954 C. Rajagopalachari An Indian independence activist, statesman, and lawyer, Rajagopalachari was the only Indian and last Governor-General of independent India. He was Chief Minister of Madras Presidency (1937–39) and Madras State (1952–54); and founder of Indian political party Swatantra Party. Sarvepalli He served as India's first Vice- Radhakrishnan President (1952–62) and second President (1962–67). Since 1962, his birthday on 5 September is observed as "Teachers' Day" in India. C. V. Raman Widely known for his work on the scattering of light and the discovery of the effect, better known as "Raman scattering", Raman mainly worked in the field of atomic physics and electromagnetism and was presented Nobel Prize in Physics in 1930. 1955 Bhagwan Das Independence activist, philosopher, and educationist, and co-founder of Mahatma Gandhi Kashi Vidyapithand worked with Madan Mohan Malaviya for the foundation of Banaras Hindu University. M. Visvesvaraya Civil engineer, statesman, and Diwan of Mysore (1912–18), was a Knight Commander of the Order of the Indian Empire. His birthday, 15 September, is observed as "Engineer's Day" in India. Jawaharlal Nehru Independence activist and author, Nehru is the first and the longest-serving Prime Minister of India (1947–64). 1957 Govind Ballabh Pant Independence activist Pant was premier of United Provinces (1937–39, 1946–50) and first Chief Minister of Uttar Pradesh (1950– 54). He served as Union Home Minister from 1955–61. 1958 Dhondo Keshav Karve Social reformer and educator, Karve is widely known for his works related to woman education and remarriage of Hindu widows. -

ISC106-Lpu- Program Schedule
TABLE OF CONTENT Sessions Page No 106th Indian Science Congress : Main Program & Plenary Session Schedule 1-5 Women Science Congress 6-7 Rashtriya Kishore Vaigyaniksammelan (Children’s Science Congress – 2019) 8-9 Vigyan Sancharak Sammelan – 2019 (Science Communicators’ Meet- 2019) 10-11 Academia- Industry Interface Session 12 Sectional Sessions & Symposium Agriculture & Forestry Sciences 13-15 Animal, Veterinary And Fishery Sciences 16-18 Anthropological And Behavioural Sciences (Including Archaeology, 21-28 Psychology, Education And Military Sciences) Chemical Sciences 29-32 Earth System Sciences 33-37 Engineering Sciences 38-41 Environmental Sciences 42-46 Information And Communication Science & Technology (Including Computer 47-48 Sciences) Materials Science 49 Mathematical Sciences (Including Statistics) 50-54 Medical Sciences 55-64 New Biology (Including Biochemistry, Biophysics & Molecular Biology And 65-70 Biotechnology) Physical Sciences 71-72 Plant Sciences 73-75 106th Indian Science Congress Programme Schedule January 03, 2019 Time: 10:00AM-01:00PM Venue- Main Pandal (Baldev Raj Mittal Unipolis) Inaugural Programme & Opening of Exhibition Time: 01:00PM-02:00PM Lunch Time: 02:00PM-03:00PM Venue- Shanti Devi Mittal Auditorium Lecture of Nobel Laureates Chair: Dr. Manoj Kumar Chakrabarti, General President, ISCA Speaker: Prof. Thomas Sudoph, NL Time: 03:00PM-04:00PM Chair(s): Prof. Ashok K.Saxena, Past General President Dr. Manoj Kumar Chakrabarti, General President Speaker: Prof. Avram Hershko, NL Time: 04:00PM-05:00PM Venue- Shanti Devi Mittal Auditorium Plenary on Futuristic Defence Technologies Chair(s): Dr. G. Satheesh Reddy, Secretary, Department of Defense R&D and Chairman, DRDO Prof. Monica Gulati, Dean, LPU & Local Secretary, ISCA Keynote Speaker: Dr. G. -
Delhi Metro Rail Corporation Ltd
DELHI METRO RAIL CORPORATION LTD. Ref: . No. DMRCs Adertiseet No.DMRC/OM/HR/I/, pulished i Eployet Nes dated 4th Sep . CBT Result otifiatio dated th Jue . th NOTICE (Dated : 17 July’ 2017) 8800 no. of candidates, bearing following Roll nos. have been short listed for the Psychological Test, based on their performance of CBT(Computer Based Test) which was held by DMRC on 21.02.17, 22.02.17, 23.02.17, 25.02.17, 27.02.17 & 28.02.17, in response to its vacancy notification No. DMRC/OM/HR/I/2016 pulished i eployet es dated: 4th Sep . Venue of Badminton Hall, DMRC Staff Quarters, Shastri Park, Delhi-110053 Document (Location- within 1Km on Kashmiri Gate ISBT-Shahdara Road on Yamuna Bridge), verification Near Shastri Park Metro Station. Venue of DMRC Training Institute, Shastri Park, Delhi-110053 Psychologica (Location- within 1Km on Kashmiri Gate ISBT-Shahdara Road on Yamuna Bridge) l Test Post Code & Customer Relations Assistant (CRA) (NE02) Name: - No. of 8800 (Including candidates belonging to reserved communities) candidates shortlisted: - Candidates are advised to attend the document verification & Psycho Test on designated dates only. Schedule for docuet verificatio & Psychological Test - Click Here Instructions for Psychological Test for the post of Customer Relations Assistant (CRA) (NE02) (Candidates are advised to check their Name / Roll No. carefully for the exact schedule of document No separate communication, calling them for document verification & Psychological Test will be made. No S. No. Roll No. Candidate Name Date of Birth -

The Year Book 2020
THE YEAR BOOK 2020 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-23616094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2020 Indian Academy of Sciences Information in this Year Book is updated up to 31 January 2020. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by The Print Point, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: -

Individual Agent List
producer Name Agreement Date Status PRATIK NAIK 01-Apr-15 Inforce BELA SHAH 01-Apr-15 Inforce YOGESH JOSHI 01-Apr-15 Inforce SUBODH KHANDELWAL 01-Apr-15 Inforce SANJAY SINGHAL 01-Apr-15 Inforce HARPREET SINGH 01-Apr-15 Inforce ANANDAN D 01-Apr-15 Inforce P.V. MOHAN BABU 01-Apr-15 Inforce J UDAY KUMAR 01-Apr-15 Inforce BABU H V RAMESH 01-Apr-15 Inforce JAISHANKAR G 01-Apr-15 Inforce G RAMANA RAO 01-Apr-15 Inforce ERUKULLA SATHYAM 01-Apr-15 Inforce VINOD KUMAR PAIDI 01-Apr-15 Inforce RANJAN KUMAR 01-Apr-15 Inforce NIMESH CURUMSEY 01-Apr-15 Inforce MAJOR FELIX MORAS 01-Apr-15 Inforce SAMIR KISHORE GANATRA 01-Apr-15 Inforce MANAS KAR 01-Apr-15 Inforce MADHURI AGARWAL 01-Apr-15 Inforce ASHOKE MUKHERJEE 01-Apr-15 Inforce DEB MITRA 01-Apr-15 Inforce RUDRESH N 01-Apr-15 Inforce HEMA PREMCHAND 01-Apr-15 Inforce VISHWAS MULGUND 01-Apr-15 Inforce NEERA SETHI 01-Apr-15 Inforce JAGAN MOHAN T M 01-Apr-15 Inforce MANJEET JUNEJA 01-Apr-15 Inforce SANJIV KHANNA 01-Apr-15 Inforce SEEMA CHANDAK 01-Apr-15 Inforce D PETER JESUDHAS 01-Apr-15 Inforce SATISH KOPPIKAR 01-Apr-15 Inforce NITIN SHAH 01-Apr-15 Inforce S SWAMINATHAN 01-Apr-15 Inforce MILIND SHAH 01-Apr-15 Inforce NIZAM SHAH 01-Apr-15 Inforce SWATI KEJRIWAL 01-Apr-15 Inforce UMESH KEDIA 01-Apr-15 Inforce M S VASANTHA KUMAR 01-Apr-15 Inforce ANANTH S 01-Apr-15 Inforce NARESH KOHLI 01-Apr-15 Inforce PANKAJ SHAH 01-Apr-15 Inforce DINESH MANGAOKAR 01-Apr-15 Inforce SANGAMESHWAR T 01-Apr-15 Inforce P VENKATESH 01-Apr-15 Inforce HEMA SHAH 01-Apr-15 Inforce T.