Scleroderma Type I Collagen in Systemic and Localized Contributes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding and Managing Scleroderma
Understanding and Managing Scleroderma A publication of Scleroderma Foundation 300 Rosewood Drive, Suite 105 Danvers, MA 01923 Maureen D. Mayes, M.D., M.P.H. Understanding and Understanding My notes and Managing Scleroderma Managing Scleroderma This booklet is intended to help people with scleroderma, their families and others interested ________________________ in learning more about the disease to better understand what scleroderma is, what effects ________________________ it may have, and what those with scleroderma can do to help themselves and their physicians ________________________ manage the disease. It answers some of the most frequently asked questions about ________________________ A publication of Maureen D. Mayes, M.D., M.P.H. Scleroderma Foundation 300 Rosewood Drive, Suite 105 scleroderma. Danvers, MA 01923 800-722-HOPE (4673) www.scleroderma.org www.facebook.com/sclerodermaUS www.twitter.com/scleroderma ________________________ Disclaimer The Scleroderma Foundation does not provide medical advice nor does it ________________________ endorse any drug or treatment mentioned herein. ________________________ The material contained in this booklet is presented for general information only. It is not intended to provide medical advice, to answer questions specific to the condition or problems of particular individuals, nor in ________________________ any way to substitute for the professional advice and care of qualified physicians. Mention of particular drugs and/or treatments is for ________________________ information purposes only and does not constitute an endorsement of said drugs and/or treatments. ________________________ Thanks! ________________________ The Scleroderma Foundation expresses its deep appreciation to the many ________________________ physicians whose efforts have led to this booklet. Special thanks are owed to Maureen D. Mayes, M.D., M.P.H., of the ________________________ University of Texas McGovern Medical School, Houston. -

The Voice of the Patient
The Voice of the Patient A series of reports from the U.S. Food and Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative Systemic Sclerosis Public Meeting: October 13, 2020 Report Date: June 30, 2021 Center for Drug Evaluation and Research (CDER) U.S. Food and Drug Administration (FDA) 1 Table of Contents Introduction ............................................................................................................................ 3 Overview of Systemic Sclerosis ................................................................................................................. 3 Meeting Overview ..................................................................................................................................... 3 Report Overview and Key Themes ............................................................................................................ 5 Topic 1: Disease Symptoms and Daily Impacts That Matter Most to Patients .......................... 6 Perspectives on Most Significant Symptoms ............................................................................................ 6 Overall Impact of Systemic Sclerosis on Daily Life .................................................................................... 9 Topic 2: Patient Perspectives on Treatments for Systemic Sclerosis ....................................... 11 Perspectives on Current Treatments ...................................................................................................... 11 Perspectives on Ideal -

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Topical Treatments for Seborrheic Keratosis: a Systematic Review
SYSTEMATIC REVIEW AND META-ANALYSIS Topical Treatments for Seborrheic Keratosis: A Systematic Review Ma. Celina Cephyr C. Gonzalez, Veronica Marie E. Ramos and Cynthia P. Ciriaco-Tan Department of Dermatology, College of Medicine and Philippine General Hospital, University of the Philippines Manila ABSTRACT Background. Seborrheic keratosis is a benign skin tumor removed through electrodessication, cryotherapy, or surgery. Alternative options may be beneficial to patients with contraindications to standard treatment, or those who prefer a non-invasive approach. Objectives. To determine the effectiveness and safety of topical medications on seborrheic keratosis in the clearance of lesions, compared to placebo or standard therapy. Methods. Studies involving seborrheic keratosis treated with any topical medication, compared to cryotherapy, electrodessication or placebo were obtained from MEDLINE, HERDIN, and Cochrane electronic databases from 1990 to June 2018. Results. The search strategy yielded sixty articles. Nine publications (two randomized controlled trials, two non- randomized controlled trials, three cohort studies, two case reports) covering twelve medications (hydrogen peroxide, tacalcitol, calcipotriol, maxacalcitol, ammonium lactate, tazarotene, imiquimod, trichloroacetic acid, urea, nitric-zinc oxide, potassium dobesilate, 5-fluorouracil) were identified. The analysis showed that hydrogen peroxide 40% presented the highest level of evidence and was significantly more effective in the clearance of lesions compared to placebo. Conclusion. Most of the treatments reviewed resulted in good to excellent lesion clearance, with a few well- tolerated minor adverse events. Topical therapy is a viable option; however, the level of evidence is low. Standard invasive therapy remains to be the more acceptable modality. Key Words: seborrheic keratosis, topical, systematic review INTRODUCTION Description of the condition Seborrheic keratoses (SK) are very common benign tumors of the hair-bearing skin, typically seen in the elderly population. -

Foot Pain in Scleroderma
Foot Pain in Scleroderma Dr Begonya Alcacer-Pitarch LMBRU Postdoctoral Research Fellow 20th Anniversary Scleroderma Family Day 16th May 2015 Leeds Institute of Rheumatic and Musculoskeletal Medicine Presentation Content n Introduction n Different types of foot pain n Factors contributing to foot pain n Impact of foot pain on Quality of Life (QoL) Leeds Institute of Rheumatic and Musculoskeletal Medicine Scleroderma n Clinical features of scleroderma – Microvascular (small vessel) and macrovascular (large vessel) damage – Fibrosis of the skin and internal organs – Dysfunction of the immune system n Unknown aetiology n Female to male ratio 4.6 : 1 n The prevalence of SSc in the UK is 8.21 per 100 000 Leeds Institute of Rheumatic and Musculoskeletal Medicine Foot Involvement in SSc n Clinically 90% of SSc patients have foot involvement n It typically has a later involvement than hands n Foot involvement is less frequent than hand involvement, but is potentially disabling Leeds Institute of Rheumatic and Musculoskeletal Medicine Different Types of Foot Pain Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Microvascular disease (small vessel) n Intermittent pain – Raynaud’s (spasm) • Cold • Throb • Numb • Tingle • Pain n Constant pain – Vessel center narrows • Distal pain (toes) • Gradually increasing pain • Intolerable pain when necrosis is present Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Macrovascular disease (large vessels) n Intermittent and constant pain – Peripheral Arterial Disease • Intermittent claudication – Muscle pain (ache, cramp) during walking • Aching or burning pain • Night and rest pain • Cramps Leeds Institute of Rheumatic and Musculoskeletal Medicine Ulcer Pain n Ulcer development – Constant pain n Infected ulcer – Unexpected/ excess pain or tenderness Leeds Institute of Rheumatic and Musculoskeletal Medicine Neuropathic Pain n Nerve damage is not always obvious. -

The Articular Manifestations of Progressive Systemic Sclerosis (Scleroderma) MURRAY BARON, PETER LEE, and EDWARD C
Ann Rheum Dis: first published as 10.1136/ard.41.2.147 on 1 April 1982. Downloaded from Annals ofthe Rheumatic Diseases, 1982, 41, 147-152 The articular manifestations of progressive systemic sclerosis (scleroderma) MURRAY BARON, PETER LEE, AND EDWARD C. KEYSTONE From the University of Toronto Rheumatic Disease Unit, The Wellesley Hospital, 160 Wellesley Street East, Toronto, Ontario, Canada M4Y 1J3 SUMMARY The articular manifestations of progressive systemic sclerosis (PSS) were studied in 38 patients. Of these, 66 % experienced joint pain and 61 % had signs of joint inflammation. Limita- tion of joint movement was seen in 45 %. Radiological abnormalities included periarticular osteoporosis (42 %), joint space narrowing (34%), and erosions (40%). Erosive disease did not correlate with disease duration, presence of rheumatoid factor, antinuclear antibodies, distal tuft resorption, or the extent of the scleroderma skin changes. Calcinosis was seen more frequently in those patients with articular erosions (67 %). Erosive osteoarthritis of the distal interphalangeal joints (7 patients) was associated with impaired finger flexion. Joint involvement in PSS occurs frequently and may resemble rheumatoid arthritis in the early stages but is less destructive. The occurrence of unrelated arthropathy, such as primary osteoarthritis, is not uncommon, and its differentiation from true PSS joint disease can be difficult. Articular manifestations are common in progressive spective study and fulfilling the preliminary diagnos- systemic sclerosis (scleroderma, PSS), and joint pain tic criteria for PSS1 were evaluated by one author is a frequent presenting feature of this disease.' Joint (M.B.). A history of joint manifestations was symptoms have been noted in 12 to 66% of patients obtained in each case according to a standard pro- http://ard.bmj.com/ at the time of diagnosis2`6 and in 24 to 97% of tocol. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Scleroderma Phenotype'' This Information Is Current As of September 27, 2021
Autoantibodies to Fibrillin-1 Activate Normal Human Fibroblasts in Culture through the TGF-β Pathway to Recapitulate the ''Scleroderma Phenotype'' This information is current as of September 27, 2021. Xiaodong Zhou, Filemon K. Tan, Dianna M. Milewicz, Xinjian Guo, Constantin A. Bona and Frank C. Arnett J Immunol 2005; 175:4555-4560; ; doi: 10.4049/jimmunol.175.7.4555 http://www.jimmunol.org/content/175/7/4555 Downloaded from References This article cites 32 articles, 11 of which you can access for free at: http://www.jimmunol.org/content/175/7/4555.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 27, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Autoantibodies to Fibrillin-1 Activate Normal Human Fibroblasts in Culture through the TGF- Pathway to Recapitulate the “Scleroderma Phenotype”1 Xiaodong Zhou,2* Filemon K. Tan,* Dianna M. -

Systemic Scleroderma
Systemic scleroderma Description Systemic scleroderma is an autoimmune disorder that affects the skin and internal organs. Autoimmune disorders occur when the immune system malfunctions and attacks the body's own tissues and organs. The word "scleroderma" means hard skin in Greek, and the condition is characterized by the buildup of scar tissue (fibrosis) in the skin and other organs. The condition is also called systemic sclerosis because the fibrosis can affect organs other than the skin. Fibrosis is due to the excess production of a tough protein called collagen, which normally strengthens and supports connective tissues throughout the body. The signs and symptoms of systemic scleroderma usually begin with episodes of Raynaud phenomenon, which can occur weeks to years before fibrosis. In Raynaud phenomenon, the fingers and toes of affected individuals turn white or blue in response to cold temperature or other stresses. This effect occurs because of problems with the small vessels that carry blood to the extremities. Another early sign of systemic scleroderma is puffy or swollen hands before thickening and hardening of the skin due to fibrosis. Skin thickening usually occurs first in the fingers (called sclerodactyly) and may also involve the hands and face. In addition, people with systemic scleroderma often have open sores (ulcers) on their fingers, painful bumps under the skin (calcinosis) , or small clusters of enlarged blood vessels just under the skin (telangiectasia). Fibrosis can also affect internal organs and can lead to impairment or failure of the affected organs. The most commonly affected organs are the esophagus, heart, lungs, and kidneys. Internal organ involvement may be signaled by heartburn, difficulty swallowing (dysphagia), high blood pressure (hypertension), kidney problems, shortness of breath, diarrhea, or impairment of the muscle contractions that move food through the digestive tract (intestinal pseudo-obstruction). -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

A Curious Keloid of the Penis
384 Letters to the Editor A Curious Keloid of the Penis Antonio Mastrolorenzo, Anna Lisa Rapaccini, Luana Tiradritti and Giuliano Zuccati Department of Dermatological Sciences, University of Florence, via Degli Alfani, 37, IT-50121 Firenze, Italy. E-mail:[email protected] Accepted April 11, 2003. Sir, performed and the histopathological analysis of the Keloids of the genitalia and penis are rare despite specimen revealed irregular and thick collagen bundles frequent surgery in this area. A careful review of the characteristic of keloid. There was no evidence of literature revealed only a few cases reported since granuloma in tissue sections to suggest a possible Browne’s statement in 1949 that the skin of the penis infectious cause. The scar was treated for the next 3 ‘‘never forms a keloid’’ (1), and Crockett’s research months with topical use of fluocinolone acetonide gel attempting to classify the susceptibility of different areas twice a day. A 12-month follow-up showed that the of the body to keloid formation and not finding any cases wound healed perfectly, leaving a small elevated, firm scar affecting genitalia in a survey of 250 Sudanese natives (2). but without itching, redness or any other sign of keloid The aim of this report is to document a case that has recurrence. In the last 6 months there was no appreciable resulted from such a common treatment as diathermy for change in the lesion. genital warts. DISCUSSION CASE REPORT We report what we believe is the tenth documented case A 32-year-old Negro man was referred to our department of keloid of the penis. -
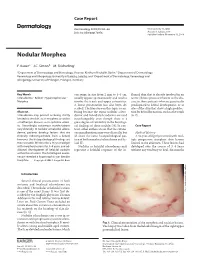
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable.