Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Topical Treatments for Seborrheic Keratosis: a Systematic Review
SYSTEMATIC REVIEW AND META-ANALYSIS Topical Treatments for Seborrheic Keratosis: A Systematic Review Ma. Celina Cephyr C. Gonzalez, Veronica Marie E. Ramos and Cynthia P. Ciriaco-Tan Department of Dermatology, College of Medicine and Philippine General Hospital, University of the Philippines Manila ABSTRACT Background. Seborrheic keratosis is a benign skin tumor removed through electrodessication, cryotherapy, or surgery. Alternative options may be beneficial to patients with contraindications to standard treatment, or those who prefer a non-invasive approach. Objectives. To determine the effectiveness and safety of topical medications on seborrheic keratosis in the clearance of lesions, compared to placebo or standard therapy. Methods. Studies involving seborrheic keratosis treated with any topical medication, compared to cryotherapy, electrodessication or placebo were obtained from MEDLINE, HERDIN, and Cochrane electronic databases from 1990 to June 2018. Results. The search strategy yielded sixty articles. Nine publications (two randomized controlled trials, two non- randomized controlled trials, three cohort studies, two case reports) covering twelve medications (hydrogen peroxide, tacalcitol, calcipotriol, maxacalcitol, ammonium lactate, tazarotene, imiquimod, trichloroacetic acid, urea, nitric-zinc oxide, potassium dobesilate, 5-fluorouracil) were identified. The analysis showed that hydrogen peroxide 40% presented the highest level of evidence and was significantly more effective in the clearance of lesions compared to placebo. Conclusion. Most of the treatments reviewed resulted in good to excellent lesion clearance, with a few well- tolerated minor adverse events. Topical therapy is a viable option; however, the level of evidence is low. Standard invasive therapy remains to be the more acceptable modality. Key Words: seborrheic keratosis, topical, systematic review INTRODUCTION Description of the condition Seborrheic keratoses (SK) are very common benign tumors of the hair-bearing skin, typically seen in the elderly population. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

A Curious Keloid of the Penis
384 Letters to the Editor A Curious Keloid of the Penis Antonio Mastrolorenzo, Anna Lisa Rapaccini, Luana Tiradritti and Giuliano Zuccati Department of Dermatological Sciences, University of Florence, via Degli Alfani, 37, IT-50121 Firenze, Italy. E-mail:[email protected] Accepted April 11, 2003. Sir, performed and the histopathological analysis of the Keloids of the genitalia and penis are rare despite specimen revealed irregular and thick collagen bundles frequent surgery in this area. A careful review of the characteristic of keloid. There was no evidence of literature revealed only a few cases reported since granuloma in tissue sections to suggest a possible Browne’s statement in 1949 that the skin of the penis infectious cause. The scar was treated for the next 3 ‘‘never forms a keloid’’ (1), and Crockett’s research months with topical use of fluocinolone acetonide gel attempting to classify the susceptibility of different areas twice a day. A 12-month follow-up showed that the of the body to keloid formation and not finding any cases wound healed perfectly, leaving a small elevated, firm scar affecting genitalia in a survey of 250 Sudanese natives (2). but without itching, redness or any other sign of keloid The aim of this report is to document a case that has recurrence. In the last 6 months there was no appreciable resulted from such a common treatment as diathermy for change in the lesion. genital warts. DISCUSSION CASE REPORT We report what we believe is the tenth documented case A 32-year-old Negro man was referred to our department of keloid of the penis. -
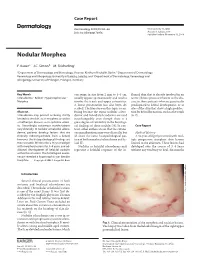
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

Nodular Scleroderma in a Patient with Chronic Hepatitis C Virus Infection: a Coexistent Or Causal Infection?
Nodular Scleroderma in a Patient With Chronic Hepatitis C Virus Infection: A Coexistent or Causal Infection? Chayada Kokpol, MD; Emily Y. Chu, MD, PhD; Suthinee Rutnin, MD PRactice Points Nodular scleroderma is a rare form of cutaneous scleroderma that can occur in association with systemic scleroderma or localized morphea. The clinical features are characterized by solitary or multiple, firm, long-lasting papules or nodules on the neck, upper trunk, and proximal extremities. The pathogenesis is still unclear. Some reports have suggested that matricellularcopy protein and growth factor, acid-fast bacteria, organic solvents, or the hepatitis C virus may be involved. not Nodular scleroderma is a rare form of scleroderma neck and trunk that had been present for 2 years. that may occur systemically or locally. The patho-DoThree years prior to presentation she had been diag- genesis of this variant is unknown. We report the nosed with systemic sclerosis (SSc) after develop- case of a 63-year-old woman with systemic sclero- ing progressive diffuse cutaneous sclerosis, Raynaud derma and chronic hepatitis C virus (HCV) infec- phenomenon with digital pitted scarring, esophageal tion who had numerous papules and nodules on dysmotility, myositis, pericardial effusion, and inter- the neck and trunk. Skin biopsies from her lesions stitial lung disease. Serologic test results were posi- revealed characteristic findings of scleroderma. tive for anti-Scl-70 antibodies. Antinuclear antibody This case not only depicts the rare entity of nodular test results were negative for anti–double-stranded scleroderma but demonstratesCUTIS the association of DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 HCV infection with systemic autoimmune diseases antibodies. -

Treatment Or Removal of Benign Skin Lesions
Treatment or Removal of Benign Skin Lesions Date of Origin: 10/26/2016 Last Review Date: 03/24/2021 Effective Date: 04/01/2021 Dates Reviewed: 10/2016, 10/2017, 10/2018, 04/2019, 10/2019, 01/2020, 03/2020, 03/2021 Developed By: Medical Necessity Criteria Committee I. Description Individuals may acquire a multitude of benign skin lesions over the course of a lifetime. Most benign skin lesions are diagnosed on the basis of clinical appearance and history. If the diagnosis of a lesion is uncertain, or if a lesion has exhibited unexpected changes in appearance or symptoms, a diagnostic procedure (eg, biopsy, excision) is indicated to confirm the diagnosis. The treatment of benign skin lesions consists of destruction or removal by any of a wide variety of techniques. The removal of a skin lesion can range from a simple biopsy, scraping or shaving of the lesion, to a radical excision that may heal on its own, be closed with sutures (stitches) or require reconstructive techniques involving skin grafts or flaps. Laser, cautery or liquid nitrogen may also be used to remove benign skin lesions. When it is uncertain as to whether or not a lesion is cancerous, excision and laboratory (microscopic) examination is usually necessary. II. Criteria: CWQI HCS-0184A Note: **If request is for treatment or removal of warts, medical necessity review is not required** A. Moda Health will cover the treatment and removal of 1 or more of the following benign skin lesions: a. Treatment or removal of actinic keratosis (pre-malignant skin lesions due to sun exposure) is considered medically necessary with 1 or more of the following procedures: i. -

Chest Scars P.27 4
DERM CASE DERM CASE Test your knowledge wTeitsht ymouulrtikpnleo-wchleodicgee cwaisthes multiple-choice cases This month – 6 cases: 1. Chest Scars p.27 4. Torso Clusters p.30 2. Redish-Brown Plaques p.28 5. Face Protrusions p.31 3. Demarcated Facial Birthmark p.29 6. Hypopigmented Papules p.32 Case 1 Chest Scars This gentleman has developed these skin lesions on his chest. He used to excessively pick his cystic acne, which was in the same area. What is your diagnosis? a. Dermatofibroma protuberans b. Keloid scar c. Perifollicular fibroma d. Angio fibroma Answer Keloid scar (answer b) , which is excessive connec - tive tissue proliferation following an injury. Therapy: No good therapy is available for Pathogenesis: Predisposing factors for keloid for - keloids. Never make any bold promises. Simple re- mation are: excision almost never works and oftentsigonifnicantly • Ethnic factors: Keloids are far more common in worsens th©e problem. ibu blacks. Treatmtent: Possibilities tinrcludead–,corticosteroid h is nlo • Location: Sternum, shoulders, neck (after iingjection, tangenltiaDl debudlkoiwng excision, and exci - yr ia an se thyroid operation), ear lobes (piercing), apnkles, sion and ccoveragerswcith skinalgruaft can be considered o er use son shins, over clavicle, edge of chin, and other sites monly if itedis certaipnetrhat the new wound can heal C ris for m tho py where skin tension is generally increaseCd. o . Auunder lcesos skin tension with exogenous pressure r ited gle • Type of injury: Burns and infections morheiboften saipnplied and that the graft donor site can be placed e pro t a form keloids, leading to aconltractusrees and prin under prophylactic pressure. -

Benign and Premalignent Skin Lesions
Benign and Premalignent Skin Lesions This pathway is about benign and premalignant skin lesions. See also Suspected Melanoma. Disclaimer Contents Disclaimer ............................................................................................................................................................................................ 1 Background .................................................................................................................................................. 2 About benign and premalignent skin lesions........................................................................................................................ 2 Assessment ................................................................................................................................................... 2 Practice Point ..................................................................................................................................................................................... 2 Management .............................................................................................................................................. 10 Premalignant lesions (actinic keratoses) ................................................................................................................................10 Benign lesions (epidermoid cysts, seborrhoeic keratoses, skin tags) .........................................................................13 Referral ...................................................................................................................................................... -

Kelsh, R.M., Mckeown-Longo, P.J. and Clark, R.A.F.: EDA Fibronectin
COMMENTARY Gauthier Y, Cario-Andre M, Lepreux S et al. (2003) Clinical Implications Melanocyte detachment after skin friction in ● In vitiligo, all the skin is affected. non lesional skin of patients with generalized vitiligo. Br J Dermatol 148:95–101 ● fi Modi cation of pigment distributions in the skin may be associated with Haass NK, Herlyn M (2005) Normal human melanocyte other alterations, such as defects in the epidermal barrier. homeostasis as a paradigm for understanding mela- noma. J Invest Dermatol Symp Proc 10:153–63 ● Stabilization of the disease, by targeting nonlesional skin, is an additional challenge for improving the benefits of treatment. Harris JE, Harris TH, Weninger W et al. (2012) A mouse model of vitiligo with focused epider- ● Full-body phototherapy may stabilize the disease by inducing the differentia- mal depigmentation requires IFN-γ for auto- + tion of melanocytes, with consequent improvements in cell–cell adhesion. reactive CD8 T-cell accumulation in the skin. JInvestDermatol132:1869–76 ● Counteracting extracellular and intracellular oxidative stress may con- Jin Y, Birlea SA, Fain PR et al. (2012) Genome-wide tribute to reducing melanocyte detachment and disease progression. association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet 44:676–80 Kovacs D, Abdel-Raouf H, Al-Khayyat M et al. (2015) Vitiligo: characterization of melano- Cario-André M, Pain C, Gauthier Y et al. (2007) The Recently, immunohistochemical examina- cytes in repigmented skin after punch grafting. melanocytorrhagic hypothesis of vitiligo tested tion of E-cadherin in tissue sam- J Eur Acad Dermatol Venereol 29:581–90 on pigmented, stressed, reconstructed epider- ples collected from patients with vitiligo, mis. -

Necrobiosis Lipoidica: Ultrastructural and Biochemical Demonstration of a Collagen Defect
Necrobiosis Lipoidica: Ultrastructural and Biochemical Demonstration of a Collagen Defect Aarne Oikarinen, M .D ., Ph. D., Minna Mo rtenhul11 cr, M .D ., M atti Kallioincn, M .D., Ph.D., and Eeva-Riitta Savolainen, M. D., Ph.D. Coll agen Resea rch Unit, Unive rsity of O Ulll , Departmcnts of Dc n1l3tology (AO, MM ), Anatomy (AO), Pathology (MK), and Medi ca l Biochcmistry (AO, E-RS), Uni ve rsity of O ulu , O Ulll , Finl and T en pati ents with necrobiosis lipoidica lesions were stud lagen was unchanged in the affected skin . Fibrobl asts ied. Five pati ents had diabetes mellitus. The age of the es tablished fr o m affected skin synthesized less coll agen than patients vari ed from 15 to 73 yea rs and the durati on o f the ce ll s deri ved fr om hea lth y-looking skin. T he decreased col skin lesions w as fro m 2 to 20 yea rs. Histologica ll y, the lagen synthesis was due to a decreased amount of m essen lesions w ere characterized by degeneration of coll agen and ger RN A fo r type I procoll agen, m easured by hybridization elas tin. In som e lesions el as tin fibers could be seen in areas w ith a specific human cDN A cl one. T he producti on of devoid of normal-looking coll agen. Electron microscopy colla genase by these fib robl as ts was not in creased. O ur revea led loss of cross-striation of coHa gen fibrils and a marked res ults thus indica te that in necro bi osis lipoidica lesions, vari atio n in the diameter o f individual coll agen fibrils.