Phylum Dinozoa – Dinoflagellates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
bioRxiv preprint doi: https://doi.org/10.1101/587352; this version posted May 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bjorbækmo et al., 23.03.2019 – preprint copy - BioRxiv The planktonic protist interactome: where do we stand after a century of research? Marit F. Markussen Bjorbækmo1*, Andreas Evenstad1* and Line Lieblein Røsæg1*, Anders K. Krabberød1**, and Ramiro Logares2,1** 1 University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N- 0316 Oslo, Norway 2 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, ES-08003, Barcelona, Catalonia, Spain * The three authors contributed equally ** Corresponding authors: Ramiro Logares: Institute of Marine Sciences (ICM-CSIC), Passeig Marítim de la Barceloneta 37-49, 08003, Barcelona, Catalonia, Spain. Phone: 34-93-2309500; Fax: 34-93-2309555. [email protected] Anders K. Krabberød: University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N-0316 Oslo, Norway. Phone +47 22845986, Fax: +47 22854726. [email protected] Abstract Microbial interactions are crucial for Earth ecosystem function, yet our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2,500 ecological interactions from ~500 publications, spanning the last 150 years. -

A Parasite of Marine Rotifers: a New Lineage of Dinokaryotic Dinoflagellates (Dinophyceae)
Hindawi Publishing Corporation Journal of Marine Biology Volume 2015, Article ID 614609, 5 pages http://dx.doi.org/10.1155/2015/614609 Research Article A Parasite of Marine Rotifers: A New Lineage of Dinokaryotic Dinoflagellates (Dinophyceae) Fernando Gómez1 and Alf Skovgaard2 1 Laboratory of Plankton Systems, Oceanographic Institute, University of Sao˜ Paulo, Prac¸a do Oceanografico´ 191, Cidade Universitaria,´ 05508-900 Butanta,˜ SP, Brazil 2Department of Veterinary Disease Biology, University of Copenhagen, Stigbøjlen 7, 1870 Frederiksberg C, Denmark Correspondence should be addressed to Fernando Gomez;´ [email protected] Received 11 July 2015; Accepted 27 August 2015 Academic Editor: Gerardo R. Vasta Copyright © 2015 F. Gomez´ and A. Skovgaard. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Dinoflagellate infections have been reported for different protistan and animal hosts. We report, for the first time, the association between a dinoflagellate parasite and a rotifer host, tentatively Synchaeta sp. (Rotifera), collected from the port of Valencia, NW Mediterranean Sea. The rotifer contained a sporangium with 100–200 thecate dinospores that develop synchronically through palintomic sporogenesis. This undescribed dinoflagellate forms a new and divergent fast-evolved lineage that branches amongthe dinokaryotic dinoflagellates. 1. Introduction form independent lineages with no evident relation to other dinoflagellates [12]. In this study, we describe a new lineage of The alveolates (or Alveolata) are a major lineage of protists an undescribed parasitic dinoflagellate that largely diverged divided into three main phyla: ciliates, apicomplexans, and from other known dinoflagellates. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

The Life Cycle of Trypanosoma Cruzi
Tyler et al. THE LIFE CYCLE OF TRYPANOSOMA CRUZI K. M. Tyler, C. L. Olson and D. M. Engman Departments of Microbiology-Immunology and Pathology Feinberg Medical School of Northwestern University, Chicago, IL 60611 ABSTRACT Since the discovery of Trypanosoma cruzi as the parasite that causes Chagas disease, nearly a century ago, the details of the organism's life cycle have fascinated scientists. T. cruzi is a single-celled eukaryote with a complex life cycle alternating between reduviid bug vectors and vertebrate hosts. It is able to adapt via the process of cellular differentiation to replicate within the diverse environments represented of the insect's gut and host cell cytoplasm. These adaptive transformations take the form of coordinated changes in morphology, metabolism and cell cycle regulation. Different life cycle stages of T. cruzi show dramatically different protein and RNA profiles, which are the end result of unusual mechanisms for regulating gene expression. In recent years, new molecular techniques have been brought to bear on the life cycle dramatically increasing our knowledge of the strategies employed by the parasite to ensure its continued survival. INTRODUCTION Chagas disease The etiologic agent of the chronic and often fatal Chagas disease is the American trypanosome, Trypanosoma cruzi , a flagellated protozoan of the order Kinetoplastida. The survival of T. cruzi is dependent on the successful transmission between, and the colonization of, two radically different environments: the midgut of the reduviid bug vector and the cytoplasm of the mammalian host cell. As is true of all infections, interruption of the pathogen's life cycle will lead to eradication of the disease. -

A PRELIMINARY NOTE on TETRAMITUS, a STAGE in the LIFE CYCLE of a COPROZOIC AMOEBA by MARTHA BUNTING
294 Z6OL6G Y.- M. BUNTING PROC. N. A. S. A PRELIMINARY NOTE ON TETRAMITUS, A STAGE IN THE LIFE CYCLE OF A COPROZOIC AMOEBA By MARTHA BUNTING DEPARTMENT OF ZOOLOGY, UNIVERSITY OF PENNSYLVANIA Communicated July 24, 1922 In 1920 a study of the Entamoeba of the rat was begun and in a culture on artificial medium a number of Coprozoic amoeba appeared, one of which exhibited a flagellate phase which seems to be identical with Tetramitus rostratus Perty.1 The appearance of flagellate phases in cultures of Coprozoic amoeba (Whitmore,2 etc.) as well as of soil amoeba (Kofoid,1 Wilson,4 etc.) has been recorded a number of times, but the flagellates which appeared were relatively simple in organization and very transitory in occurrence. Fur- thermore, some of the simpler flagellates have been observed (Pascher5) to lose their flagella and become amoeboid. In the case here described, however, there is a transformation of a simple amoeba into a relatively complex flagellate which multiplies for several days then returns to the amoeboid condition and becomes encysted. Tetramitus rostratus has been studied by a number of investigators since its discovery in 1852, by Perty,' yet no one has given a full account of its life history. The lack of cysts in previous accounts, mentioned by Dobell and O'Connor,6 is explained by the transformation into an amoeba before encystment. It is believed that this animal presents the extreme in transformations of this sort and serves to emphasize the close relationship between amoebae and flagellates, and the need for careful studies of life cycles, in pure cultures where possible, in investigations of coprozoic and other Protozoa. -

Chapter 6.2-Assessment of Harmful Algae Bloom
Maryland’s Coastal Bays: Ecosystem Health Assessment Chapter 6.2 Chapter 6.2 Assessment of harmful algae bloom species in the Maryland Coastal Bays Catherine Wazniak Maryland Department of Natural Resources, Tidewater Ecosystem Assessment, Annapolis, MD 21401 Abstract Thirteen potentially harmful algae taxa have been identified in the Maryland Coastal Bays: Aureococcus anophagefferens (brown tide), Pfiesteria piscicida and P. shumwayae, Chloromorum/ Chattonella spp., Heterosigma akashiwo, Fibrocapsa japonica, Prorocentrum minimum, Dinophysis spp., Amphidinium spp., Pseudo-nitzchia spp., Karlodinium micrum and two macroalgae genera (Gracilaria, Chaetomorpha). Presence of potentially toxic species is richest in the polluted tributaries of St. Martin River and Newport Bay. Approximately 5% of the phytoplankton species identified for Maryland’s Coastal Bays represent potentially harmful algal bloom (HAB) species. The HABs are recognized for their potentially toxic properties and, in some cases, their ability to produce large blooms negatively affecting light and dissolved oxygen resources. Brown tide (Aureococcus anophagefferens) has been the most widespread and prolific HAB species in the area in recent years, producing growth impacts to juvenile clams in test studies and potential impacts to sea grass distribution and growth (see Chapter 7.1). Macroalgal fluctuations may be evidence of a system balancing on the edge of a eutrophic (nutrient- enriched) state (see chapter 4). No evidence of toxic activity has been detected among the Coastal Bays phytoplankton. However, species such as Pseudo-nitzschia seriata, Prorocentrum minimum, Pfiesteria piscicida, Dinophysis acuminata and Karlodinium micrum have produced positive toxic bioassays or generated detectable toxins in Chesapeake Bay. Pfiesteria piscicida was retrospectively considered as the likely causative organism in a large historical fish kill on the Indian River, Delaware. -

Worms, Germs, and Other Symbionts from the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor
h ' '' f MASGC-B-78-001 c. 3 A MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico CRCDU7M COPY Sea Grant Depositor NATIONAL SEA GRANT DEPOSITORY \ PELL LIBRARY BUILDING URI NA8RAGANSETT BAY CAMPUS % NARRAGANSETT. Rl 02882 Robin M. Overstreet r ii MISSISSIPPI—ALABAMA SEA GRANT CONSORTIUM MASGP—78—021 MARINE MALADIES? Worms, Germs, and Other Symbionts From the Northern Gulf of Mexico by Robin M. Overstreet Gulf Coast Research Laboratory Ocean Springs, Mississippi 39564 This study was conducted in cooperation with the U.S. Department of Commerce, NOAA, Office of Sea Grant, under Grant No. 04-7-158-44017 and National Marine Fisheries Service, under PL 88-309, Project No. 2-262-R. TheMississippi-AlabamaSea Grant Consortium furnish ed all of the publication costs. The U.S. Government is authorized to produceand distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon. Copyright© 1978by Mississippi-Alabama Sea Gram Consortium and R.M. Overstrect All rights reserved. No pari of this book may be reproduced in any manner without permission from the author. Primed by Blossman Printing, Inc.. Ocean Springs, Mississippi CONTENTS PREFACE 1 INTRODUCTION TO SYMBIOSIS 2 INVERTEBRATES AS HOSTS 5 THE AMERICAN OYSTER 5 Public Health Aspects 6 Dcrmo 7 Other Symbionts and Diseases 8 Shell-Burrowing Symbionts II Fouling Organisms and Predators 13 THE BLUE CRAB 15 Protozoans and Microbes 15 Mclazoans and their I lypeiparasites 18 Misiellaneous Microbes and Protozoans 25 PENAEID -

The Eukaryotic Life on Microplastics in Brackish Ecosystems
Mathematisch-Naturwissenschaftliche Fakultät Marie Therese Kettner | Sonja Oberbeckmann | Matthias Labrenz Hans-Peter Grossart The Eukaryotic Life on Microplastics in Brackish Ecosystems Suggested citation referring to the original publication: Frontiers in Microbiology 10 (2019), Art. 538 DOI https://doi.org/10.3389/fpsyg.2015.00766 ISSN (online) 1664-302X Postprint archived at the Institutional Repository of the Potsdam University in: Postprints der Universität Potsdam Mathematisch-Naturwissenschaftliche Reihe ; 741 ISSN 1866-8372 https://nbn-resolving.org/urn:nbn:de:kobv:517-opus4-434996 DOI https://doi.org/10.25932/publishup-43499 fmicb-10-00538 March 19, 2019 Time: 17:29 # 1 ORIGINAL RESEARCH published: 20 March 2019 doi: 10.3389/fmicb.2019.00538 The Eukaryotic Life on Microplastics in Brackish Ecosystems Marie Therese Kettner1,2, Sonja Oberbeckmann3, Matthias Labrenz3 and Hans-Peter Grossart1,2* 1 Department of Experimental Limnology, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Berlin, Germany, 2 Institute for Biochemistry and Biology, University of Potsdam, Potsdam, Germany, 3 Environmental Microbiology Working Group, Leibniz Institute for Baltic Sea Research Warnemünde, Rostock, Germany Microplastics (MP) constitute a widespread contaminant all over the globe. Rivers and wastewater treatment plants (WWTP) transport annually several million tons of MP into freshwaters, estuaries and oceans, where they provide increasing artificial surfaces for microbial colonization. As knowledge on MP-attached communities is insufficient for brackish ecosystems, we conducted exposure experiments in the coastal Baltic Sea, an in-flowing river and a WWTP within the drainage basin. While reporting on prokaryotic and fungal communities from the same set-up previously, we focus here on the entire eukaryotic communities. -

A Monograph on the Protozoa of the Large Intestine of the Horse Ta-Shih Hsiung Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1930 A monograph on the protozoa of the large intestine of the horse Ta-Shih Hsiung Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Zoology Commons Recommended Citation Hsiung, Ta-Shih, "A monograph on the protozoa of the large intestine of the horse" (1930). Retrospective Theses and Dissertations. 14768. https://lib.dr.iastate.edu/rtd/14768 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overiaps. -
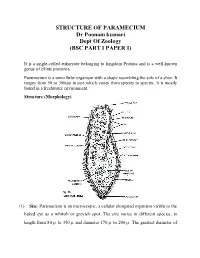
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Infectious Diseases of Laboratory Zebrafish
INFECTIOUS DISEASES OF LABORATORY ZEBRAFISH Justin L. Sanders Department of Biomedical Sciences Infectious Diseases – ZIRC Diagnostic Service Summaries 2006-2014 About 7,000 fish and 100 facilities • Pseudoloma : 48% labs, 16% fish • Mycobacteriosis: 40% labs, 6% fish • Edwardsiella ictaluri: 3 labs in 2011. • Pseudocapillaria: 11% labs, 1.3% fish • Gut Hyperplasia/Neoplasia: 12% labs, 2.8% fish • Myxidium: 19% labs, 2.4% fish • Piscinoodinium (max 2 %), Pleistophora (max 7%) Pathogens in Zebrafish Facilities 2006-2015: – ca 10,000 fish, 100 laboratories Parasitic Diseases: general pathology • Space replacement/pressure atrophy • Respiratory impediment – gill parasites • Osmoregulatory – gill & skin infections • Perforation of gut & 2 nd bacterial infections • Inflammatory changes Common parasites of research zebrafish Parasite Group Species Reference Dinoflagellate Piscinoodinium pillulare Westerfield 2000 Ciliate Ichthyophthirius multifiliis Matthews 2004 Nematode Pseudocapillaria tomentosa Kent et al. 2002 Myxozoan Myxidium streisingeri Whipps et al. 2015 Digenea Transversotrema patialense Womble et al. 2015 Microsporidia Pseudoloma neurophilia de Kinkelin 1980; Matthews et al. 2001 Pleistophora Sanders et al. 2010 hyphessobryconis Myxidium streisingeri Whipps et al 2015 Myxidium streisingeri Whipps et al 2015 Myxidium streisingeri Whipps et al 2015 Myxidium streisingeri Pathogenesis Mode of Diagnosis Control/Treament transmission Found in lumen Unknown: Wet mount: light Avoidance/quarantine of mesonephric microscopy; and kidney Direct? -

Proceedings of the 40Th U.S.-Japan Aquaculture Panel Symposium
Hatchery Technology for High Quality Juvenile Production Proceedings of the 40th U.S.-Japan Aquaculture Panel Symposium University of Hawaii East West Center Honolulu, Hawaii October 22-23 2012 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service NOAA Technical Memorandum NMFS-F/SPO-136 Hatchery Technology for High Quality Juvenile Production Proceedings of the 40th U.S.-Japan Aquaculture Panel Symposium University of Hawaii East West Center Honolulu, Hawaii October 22-23 2012 Mike Rust1, Paul Olin2, April Bagwill3, and Marie Fujitani3, editors 1Northwest Fisheries Science Center 2725 Montlake Boulevard East Seattle, Washington 98112 2California Sea Grant UCSD / Scripps Institution of Oceanography 133 Aviation Blvd., Suite 109 Santa Rosa CA 95403 3NOAA National Marine Fisheries Service 1315 East-West Highway Silver Spring, MD 20910 NOAA Technical Memorandum NMFS-F/SPO-136 December 2013 U.S. Department of Commerce Penny Pritzker, Secretary of Commerce National Oceanic and Atmospheric Administration Dr. Kathryn Sullivan, (Acting) NOAA Administrator National Marine Fisheries Service Samuel D. Rauch III, (Acting) Assistant Administrator for Fisheries SUGGESTED CITATION: Rust, M., P. Olin, A. Bagwill and M. Fujitani (editors). 2013. Hatchery Technology for High Quality Juvenile Production: Proceedings of the 40th U.S.-Japan Aquaculture Panel Symposium, Honolulu, Hawaii, October 22-23, 2012. U.S. Dept. Commerce, NOAA Tech. Memo. NMFS-F/SPO-136. A COPY OF THIS REPORT MAY BE OBTAINED FROM: Northwest Fisheries Science Center 2725 Montlake Boulevard East Seattle, Washington 98112 OR ONLINE AT: http://spo.nmfs.noaa.gov/tm/ Reference throughout this document to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA.