Impacts of Reduced Lepidurus Arcticus Availability on Brown Trout Life History Traits in a Mountain Reservoir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Fig. Ap. 2.1. Denton Tending His Fairy Shrimp Collection
Fig. Ap. 2.1. Denton tending his fairy shrimp collection. 176 Appendix 1 Hatching and Rearing Back in the bowels of this book we noted that However, salts may leach from soils to ultimately if one takes dry soil samples from a pool basin, make the water salty, a situation which commonly preferably at its deepest point, one can then "just turns off hatching. Tap water is usually unsatis- add water and stir". In a day or two nauplii ap- factory, either because it has high TDS, or because pear if their cysts are present. O.K., so they won't it contains chlorine or chloramine, disinfectants always appear, but you get the idea. which may inhibit hatching or kill emerging If your desire is to hatch and rear fairy nauplii. shrimps the hi-tech way, you should get some As you have read time and again in Chapter 5, guidance from Brendonck et al. (1990) and temperature is an important environmental cue for Maeda-Martinez et al. (1995c). If you merely coaxing larvae from their dormant state. You can want to see what an anostracan is like, buy some guess what temperatures might need to be ap- Artemia cysts at the local aquarium shop and fol- proximated given the sample's origin. Try incu- low directions on the container. Should you wish bation at about 3-5°C if it came from the moun- to find out what's in your favorite pool, or gather tains or high desert. If from California grass- together sufficient animals for a study of behavior lands, 10° is a good level at which to start. -

Freshwater Crustaceans As an Aboriginal Food Resource in the Northern Great Basin
UC Merced Journal of California and Great Basin Anthropology Title Freshwater Crustaceans as an Aboriginal Food Resource in the Northern Great Basin Permalink https://escholarship.org/uc/item/3w8765rq Journal Journal of California and Great Basin Anthropology, 20(1) ISSN 0191-3557 Authors Henrikson, Lael S Yohe, Robert M, II Newman, Margaret E et al. Publication Date 1998-07-01 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Joumal of Califomia and Great Basin Anthropology Vol. 20, No. 1, pp. 72-87 (1998). Freshwater Crustaceans as an Aboriginal Food Resource in the Northern Great Basin LAEL SUZANN HENRIKSON, Bureau of Land Management, Shoshone District, 400 W. F Street, Shoshone, ID 83352. ROBERT M. YOHE II, Archaeological Survey of Idaho, Idaho State Historical Society, 210 Main Street, Boise, ID 83702. MARGARET E. NEWMAN, Dept. of Archaeology, University of Calgary, Alberta, Canada T2N 1N4. MARK DRUSS, Idaho Power Company, 1409 West Main Street, P.O. Box 70. Boise, ID 83707. Phyllopods of the genera Triops, Lepidums, and Branchinecta are common inhabitants of many ephemeral lakes in the American West. Tadpole shrimp (Triops spp. and Lepidums spp.) are known to have been a food source in Mexico, and fairy shrimp fBranchinecta spp.) were eaten by the aborigi nal occupants of the Great Basin. Where found, these crustaceans generally occur in numbers large enough to supply abundant calories and nutrients to humans. Several ephemeral lakes studied in the Mojave Desert arul northern Great Basin currently sustain large seasonal populations of these crusta ceans and also are surrounded by numerous small prehistoric camp sites that typically contain small artifactual assemblages consisting largely of milling implements. -

VERNAL POOL TADPOLE SHRIMP Lepidurus Packardi
U.S. Fish & Wildlife Service Sacramento Fish & Wildlife Office Species Account VERNAL POOL TADPOLE SHRIMP Lepidurus packardi CLASSIFICATION: Endangered Federal Register 59-48136; September 19, 1994 http://ecos.fws.gov/docs/federal_register/fr2692.pdf On October 9, 2007, we published a 5-year review recommending that the species remain listed as endangered. CRITICAL HABITAT: Designated Originally designated in Federal Register 68:46683; August 6, 2003. The designation was revised in FR 70:46923; August 11, 2005. Species by unit designations were published in FR 71:7117 (PDF), February 10, 2006. RECOVERY PLAN: Final Recovery Plan for Vernal Pool Ecosystems of California and Southern Oregon, December 15, 2005. http://ecos.fws.gov/docs/recovery_plan/060614.pdf DESCRIPTION The vernal pool tadpole shrimp (Lepidurus packardi) is a small crustacean in the Triopsidae family. It has compound eyes, a large shield-like carapace (shell) that covers most of the body, and a pair of long cercopods (appendages) at the end of the last abdominal segment. Vernal pool tadpole shrimp adults reach a length of 2 inches in length. They have about 35 pairs of legs and two long cercopods. This species superficially resembles the rice field tadpole shrimp (Triops longicaudatus). Tadpole shrimp climb or scramble over objects, as well as plowing along or within bottom sediments. Their diet consists of organic debris and living organisms, such as fairy shrimp and other invertebrates. This animal inhabits vernal pools containing clear to highly turbid water, ranging in size from 54 square feet in the former Mather Air Force Base area of Sacramento County, to the 89-acre Olcott Lake at Jepson Prairie. -

Crustacea, Notostraca) from Iran
Journal of Biological Research -Thessaloniki 19 : 75 – 79 , 20 13 J. Biol. Res. -Thessalon. is available online at http://www.jbr.gr Indexed in: Wos (Web of science, IsI thomson), sCOPUs, CAs (Chemical Abstracts service) and dOAj (directory of Open Access journals) — Short CommuniCation — on the occurrence of Lepidurus apus (Linnaeus, 1758) (Crustacea, notostraca) from iran Behroz AtAshBAr 1,2 *, N aser Agh 2, L ynda BeLAdjAL 1 and johan MerteNs 1 1 Department of Biology , Faculty of Science , Ghent University , K.L. Ledeganckstraat 35 , 9000 Gent , Belgium 2 Department of Biology and Aquaculture , Artemia and Aquatic Animals Research Institute , Urmia University , Urmia-57153 , Iran received: 16 November 2011 Accepted after revision: 18 july 2012 the occurrence of Lepidurus apus in Iran is reported for the first time. this species was found in Aigher goli located in the mountains area, east Azerbaijan province (North east, Iran). details on biogeography, ecology and morphology of this species are provided. Key words: Lepidurus apus , Aigher goli, east Azerbaijan, Iran. INtrOdUCtION their world-wide distribution is due to their anti qu - ity, but possibly also to their passive transport: geo - Notostracan records date back to the Carboniferous graphical barriers are more effective for non-passive - and possibly up to the devonian period (Wallossek, ly distributed animals. From an ecological point of 1993, 1995; Kelber, 1998). In fact, there are Upper view, notostracans, like most branchiopods, are re - triassic Triops fossils from germany -

Lepidurus Arcticus (Crustacea : Notostraca); an Unexpected Prey of Arctic Charr (Salvelinus Alpinus) in a High Arctic River
BOREAL ENVIRONMENT RESEARCH 23: 149–157 © 2018 ISSN 1797-2469 (online) Helsinki 20 April 2018 Lepidurus arcticus (Crustacea : Notostraca); an unexpected prey of Arctic charr (Salvelinus alpinus) in a High Arctic river Reidar Borgstrøm1)*, Morten Aas1)2), Hanne Hegseth1)2), J. Brian Dempson3) and Martin-Arne Svenning2) 1) Faculty of Environmental Sciences and Natural Resource Management, Norwegian University of Life Sciences, P.O. Box 5003, NO-1432 Ås, Norway (*corresponding author’s e-mail: reidar.borgstrom@ nmbu.no) 2) Norwegian Institute of Nature Research, Arctic Ecology Department, Fram Center, P.O. Box 6606 Langnes, NO-9296 Tromsø, Norway 3) Fisheries & Oceans Canada, Science Branch, 80 East White Hills Road, P.O. Box 5667, St. John’s, NL A1C 5X1, Canada Received 20 Dec. 2017, final version received 16 Mar. 2018, accepted 12 Apr. 2018 Borgstrøm R., Aas M., Hegseth H., Dempson J.B. & Svenning M.-A. 2018: Lepidurus arcticus (Crusta- cea : Notostraca); an unexpected prey of Arctic charr (Salvelinus alpinus) in a High Arctic river. Boreal Env. Res. 23: 149–157. The phyllopod Lepidurus arcticus, commonly called the arctic tadpole shrimp, is an important food item of both Arctic charr Salvelinus alpinus and brown trout Salmo trutta in lakes in Iceland and Scandinavia, especially at low fish densities. In the High Arctic, the tadpole shrimp is abundant in fishless localities, but absent or rare in most lakes where fish are present. We studied the diet of Arctic charr in the Straumsjøen watercourse on Spitsbergen, the main island of Svalbard. The outlet river is dry nine months of the year, and all Arctic charr present during summer have descended from the lake. -

Crustacea: Notostraca) from Serbia
Arthropoda Selecta 28(1): 65–72 © ARTHROPODA SELECTA, 2019 Developmental and other body abnormalities in the genus Lepidurus Leach, 1819 (Crustacea: Notostraca) from Serbia Îòêëîíåíèÿ â ðàçâèòèè è äðóãèå íàðóøåíèÿ ñòðîåíèÿ òåëà â ðîäå Lepidurus Leach, 1819 (Crustacea: Notostraca) â Ñåðáèè Ivana Šaganović, Slobodan Makarov, Luka Lučić, Sofija Pavković- Lučić, Vladimir Tomić, Dragana Miličić Èâàíà Øàãàíîâèћ, Ñëîáîäàí Ìàêàðîâ, Ëóêà Ëó÷èћ, Ñîôè¼à Ïàâêîâèћ-Ëó÷èћ, Âëàäèìèð Òîìèћ, Äðàãàíà Ìèëè÷èћ University of Belgrade, Faculty of Biology, Institute of Zoology, Studentski Trg 16, 11000 Belgrade, Serbia. Университет Белграда, Биологический факультет, Институт зоологии, Белград, Сербия. KEY WORDS: morphological abnormalities, Lepidurus, Serbia. КЛЮЧЕВЫЕ СЛОВА: морфологические отклонения, Lepidurus, Сербия. ABSTRACT. The occurrence of morphological ab- шние изменения в строении тела у крупных рако- normalities and the emergence of body defects are образных-бранхиопод рода Lepidurus Leach, 1819 well-known phenomena in crustaceans and other groups (Notostraca), собранных в Сербии, и обсудить их of arthropods. They usually originate from genetic fac- возможную этиологию. Большинство изменений tors and are attributable to aberrations during the moult- отмечены у карапакса и конечностей, а затем у ing process, but they can also be of exogenous origin. брюшного отдела, тельсона с хвостовой долей и у The aims of this study were to present the external церкоподов. Что касается потенциальных причин, body changes that occurred in large branchiopod crus- наблюдаемые изменения могут вызываться как ге- taceans of the genus Lepidurus Leach, 1819 (Notostra- нетическими, так и негенетическими факторами ca) sampled in Serbia and discuss their possible etiolo- типа пресс хищников и возможные нарушения при- gy. The majority of variations were identified on the родной среды. -

Crustacean Phylogeny…? Nauplius • First Larva Stage of Most “It Can Be Concluded That Crustacean Crustaceans
Bio 370 Crustacea Main arthropod clades (Regier et al 2010) Phylum Arthropoda http://blogs.discoverm • Trilobita agazine.com/loom/201 0/02/10/blind-cousins- Subphylum (or Class) Crustacea to-the-arthropod- • Chelicerata superstars/ Mostly aquatic, with calcified exoskeleton. • Mandibulata – Myriapoda (Chilopoda, Diplopoda) Head derived from acron plus next five segments- so primitively has 5 pairs of appendages: – Pancrustacea • Oligostraca (Ostracoda, Branchiura) -2 pair antennae • Altocrustacea - 1 pair of jaws – Vericrustacea - 2 pair of maxillae » (Branchiopoda, Decapoda) - usually a median (cyclopean) eye and – Miracrustacea one pair of compound eyes » Xenocarida (Remipedia, Cephalocarida) » Hexapoda Tagmosis of trunk varies in different taxa Crustacean phylogeny…? Nauplius • first larva stage of most “It can be concluded that crustacean crustaceans. phylogeny remains essentially unresolved. • three pairs of appendages • single median (naupliar) eye Conflict is rife, irrespective of whether one compares different morphological studies, molecular studies, or both.” Appendages: Jenner, 2010: Arthropod Structure & Development 39:143– -1st antennae 153 -2nd antennae - mandibles 1 Bio 370 Crustacea Crustacean taxa you should know Remipede habitat: a sea cave “blue hole” on Andros Island. Seven species are found in the Bahamas. Class Remipedia Class Malacostraca Class Branchiopoda “Peracarida”-marsupial crustacea Notostraca –tadpole shrimp Isopoda- isopods Anostraca-fairy shrimp Amphipoda- amphipods Cladocera- water fleas Mysidacea- mysids Conchostraca- clam shrimp “Eucarida” Class Maxillopoda Euphausiacea- krill Ostracoda- ostracods Decapoda- decapods- ten leggers Copepoda- copepods Branchiura- fish lice Penaeoidea- penaeid shrimp Cirripedia- barnacles Caridea- carid shrimp Astacidea- crayfish & lobsters Brachyura- true crabs Anomura- false crabs “Stomatopoda”– mantis shrimps Class Remipedia Remipides found only in sea caves in the Caribbean, the Canary Islands, and Western Australia (see pink below). -
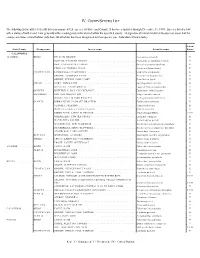
Iv. County/Species List
IV. COUNTY/SPECIES LIST The following list identifies federally listed or proposed U.S. species by State and County. It has been updated through December 31, 1999. Species listed below with a status of both E and T are generally either endangered or threatened within the specified county. Designation of critical habitat (CH) does not mean that the county constitutes critical habitat, only that critical habitat has been designated for that species (see Addendum A Instructions). Action/ State/County Group name Inverse name Scientific name Status CALIFORNIA ALAMEDA ......... BIRDS ....... PELICAN, BROWN ........................................ Pelicanus occidentalis E PLOVER, WESTERN SNOWY ................................ Charadrius alexandrinus nivosus T RAIL, CALIFORNIA CLAPPER ............................... Rallus longirostris obsoletus E TERN, CALIFORNIA LEAST ................................. Sterna antillarum browni E CRUSTACEAN . LINDERIELLA, CALIFORNIA ................................ Linderiella occidentalis E SHRIMP, LONGHORN FAIRY ................................ Branchinecta longiantenna E SHRIMP, VERNAL POOL FAIRY .............................. Branchinecta lynchi T FISHES ...... GOBY, TIDEWATER ...................................... Eucyclogobius newberryi E SPLITTAIL, SACRAMENTO ................................. Pogonichthys macrolepidotus T INSECTS ..... BUTTERFLY, BAY CHECKERSPOT ........................... Euphydryas editha bayensis T MAMMALS ... FOX, SAN JOAQUIN KIT .................................... Vulpes macrotis -

F. Christian Thompson Neal L. Evenhuis and Curtis W. Sabrosky Bibliography of the Family-Group Names of Diptera
F. Christian Thompson Neal L. Evenhuis and Curtis W. Sabrosky Bibliography of the Family-Group Names of Diptera Bibliography Thompson, F. C, Evenhuis, N. L. & Sabrosky, C. W. The following bibliography gives full references to 2,982 works cited in the catalog as well as additional ones cited within the bibliography. A concerted effort was made to examine as many of the cited references as possible in order to ensure accurate citation of authorship, date, title, and pagination. References are listed alphabetically by author and chronologically for multiple articles with the same authorship. In cases where more than one article was published by an author(s) in a particular year, a suffix letter follows the year (letters are listed alphabetically according to publication chronology). Authors' names: Names of authors are cited in the bibliography the same as they are in the text for proper association of literature citations with entries in the catalog. Because of the differing treatments of names, especially those containing articles such as "de," "del," "van," "Le," etc., these names are cross-indexed in the bibliography under the various ways in which they may be treated elsewhere. For Russian and other names in Cyrillic and other non-Latin character sets, we follow the spelling used by the authors themselves. Dates of publication: Dating of these works was obtained through various methods in order to obtain as accurate a date of publication as possible for purposes of priority in nomenclature. Dates found in the original works or by outside evidence are placed in brackets after the literature citation. -

United States Department of the Interior
United States Department of the Interior FISH AND WILDLIFE SERVICE Pacific Southwest Region 2800 Cottage Way, Suite W-2606 Sacramento, California 95825-1846 IN REPLY REFER TO: May 31, 2015 Survey Guidelines for the Listed Large Branchiopods Introduction The endangered Conservancy fairy shrimp (Branchinecta conservatio), longhorn fairy shrimp (Branchinecta longiantenna), vernal pool tadpole shrimp (Lepidurus packardi), and the threatened vernal pool fairy shrimp (Branchinecta lynchi) were listed on September 19, 1994, under the Endangered Species Act of 1973, as amended (Act) (59 Federal Register 48136). These species are endemic to vernal pools in the Agate Desert Region of Oregon and in California’s Central Valley, Coast Ranges, and a limited number of sites in and south of the Transverse Ranges. The endangered Riverside fairy shrimp (Streptocephalus woottoni) was listed under the Act on August 3, 1993 (58 Federal Register 41391). This species inhabits Riverside, Orange, Los Angeles, and San Diego counties, California, and northwestern Baja California, Mexico. The San Diego fairy shrimp (Branchinecta sandiegonensis) was listed under the Act on February 3, 2007 (62 Federal Register 4925). This species inhabits San Diego and Orange counties in California, and northwestern Baja California, Mexico. These six species, hereafter referred to as the listed large branchiopods, are fully protected under the Act. Surveys for all of these species should follow the methods described in these Survey Guidelines for the Listed Large Branchiopods (Guidelines). It is expected that the Guidelines will be revised in the future as additional information becomes available. The U.S. Fish and Wildlife Service (Service) has published recovery plans for vernal pool species in Southern California (Service 1998a) and in Northern California and Southern Oregon (Service 2005, 2012). -

A ''Crown of Thorns'' Is an Inducible Defense That Protects Daphnia Against an Ancient Predator
A ‘‘crown of thorns’’ is an inducible defense that protects Daphnia against an ancient predator Adam Petruseka,1, Ralph Tollrianb, Klaus Schwenkc, Andreas Haasd, and Christian Laforschd,1,2 aDepartment of Ecology, Faculty of Science, Charles University, Vinicˇna´ 7, CZ-12844 Prague, Czech Republic; bAnimal Ecology, Evolution and Biodiversity, Ruhr-University Bochum, Universita¨tsstrasse 150 /ND05, D-44780 Bochum, Germany; cDepartment of Ecology and Evolution, Johann Wolfgang Goethe-University, Siesmayerstrasse 70, D-60054 Frankfurt am Main, Germany; and dDepartments of Biology II and Evolutionary Ecology, and GeoBio-Center, Ludwig-Maximilians University Munich, Grosshaderner Strasse 2, D-82152 Planegg-Martinsried, Germany Edited by May R. Berenbaum, University of Illinois at Urbana-Champaign, Urbana, IL, and approved December 12, 2008 (received for review August 14, 2008) Genetic data has become an essential part of ecological studies, Members of the Daphnia atkinsoni complex are typical for the because the analyses of diversity within and among natural pop- Mediterranean region, although the range of the species complex ulations may grant access to previously overlooked ecological and extends to Central Asia, North-West Europe, and even Iceland evolutionary causalities, especially among cryptic species. Here, we and Greenland. A characteristic morphological feature shared by present an example of how phylogenetic analysis of molecular these Daphnia is a dorsal extension of the carapace into the head data obtained within a DNA barcoding study, in combination with shield that forms a heart-shaped lobe (Fig. 1). In some popula- morphological and ecological data from the field and laboratory tions, these lobes cover a large portion of the head and are armed experiments, unraveled a striking predator-prey interaction be- by long spines on the edge, forming a veritable ‘‘crown of tween aquatic organisms. -

“Living Fossil“ Crustacean Order of the Notostraca
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Open Marine Archive OPEN 3 ACCESS Freely available online tlos one Toward a Global Phylogeny of the “Living Fossil“ Crustacean Order of the Notostraca Bram Vanschoenwinkel1*9, Tom Pinceel19, Maarten P. M. Vanhove2, Carla Denis1, Merlijn Jocque3, Brian V. Timms4, Luc Brendonck1 1 Laboratory of Aquatic Ecology and Evolutionary Biology, KU Leuven, Leuven, Belgium, 2 Laboratory of Animal Diversity and Systematics, KU Leuven, Leuven, Belgium, 3 Royal Belgian Institute of Natural Sciences, Brussels, Belgium, 4 Australian Museum, Sydney, New South Wales, Australia Abstract Tadpole shrimp (Crustacea, Notostraca) are iconic inhabitants of temporary aquatic habitats worldwide. Often cited as prime examples of evolutionary stasis, surviving representatives closely resemble fossils older than 200 mya, suggestive of an ancient origin. Despite significant interest in the group as 'living fossils' the taxonomy of surviving taxa is still under debate and both the phylogenetic relationships among different lineages and the timing of diversification remain unclear. We constructed a molecular phylogeny of the Notostraca using model based phylogenetic methods. Our analyses supported the monophyly of the two generaTriops andLepidurus, although forTriops support was weak. Results also revealed high levels of cryptic diversity as well as a peculiar biogeographic link between Australia and North America presumably mediated by historic long distance dispersal. We concluded that, although some present day tadpole shrimp species closely resemble fossil specimens as old as 250 mya, no molecular support was found for an ancient (pre) Mesozoic radiation. Instead, living tadpole shrimp are most likely the result of a relatively recent radiation in the Cenozoic era and close resemblances between recent and fossil taxa are probably the result of the highly conserved general morphology in this group and of homoplasy.