Coccolithophore Community Response to Ocean Acidification And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Interactions Between Phytoplankton and Microzooplankton: Their Influence on the Coupling Between Growth and Grazing Rates in the Sea
Hydrobiologia 480: 41–54, 2002. 41 C.E. Lee, S. Strom & J. Yen (eds), Progress in Zooplankton Biology: Ecology, Systematics, and Behavior. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Novel interactions between phytoplankton and microzooplankton: their influence on the coupling between growth and grazing rates in the sea Suzanne Strom Shannon Point Marine Center, Western Washington University, 1900 Shannon Point Rd., Anacortes, WA 98221, U.S.A. Tel: 360-293-2188. E-mail: [email protected] Key words: phytoplankton, microzooplankton, grazing, stability, behavior, evolution Abstract Understanding the processes that regulate phytoplankton biomass and growth rate remains one of the central issues for biological oceanography. While the role of resources in phytoplankton regulation (‘bottom up’ control) has been explored extensively, the role of grazing (‘top down’ control) is less well understood. This paper seeks to apply the approach pioneered by Frost and others, i.e. exploring consequences of individual grazer behavior for whole ecosystems, to questions about microzooplankton–phytoplankton interactions. Given the diversity and paucity of phytoplankton prey in much of the sea, there should be strong pressure for microzooplankton, the primary grazers of most phytoplankton, to evolve strategies that maximize prey encounter and utilization while allowing for survival in times of scarcity. These strategies include higher grazing rates on faster-growing phytoplankton cells, the direct use of light for enhancement of protist digestion rates, nutritional plasticity, rapid population growth combined with formation of resting stages, and defenses against predatory zooplankton. Most of these phenomena should increase community-level coupling (i.e. the degree of instantaneous and time-dependent similarity) between rates of phytoplankton growth and microzooplankton grazing, tending to stabilize planktonic ecosystems. -

Overcalcified Forms of the Coccolithophore Emiliania Huxleyi in High CO2 Waters Are Not Pre-Adapted to Ocean Acidification
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-303 Manuscript under review for journal Biogeosciences Discussion started: 6 September 2017 c Author(s) 2017. CC BY 4.0 License. Overcalcified forms of the coccolithophore Emiliania huxleyi in high CO2 waters are not pre-adapted to ocean acidification. Peter von Dassow1,2,3*, Francisco Díaz-Rosas1,2, El Mahdi Bendif4, Juan-Diego Gaitán-Espitia5, Daniella Mella-Flores1, Sebastian Rokitta6, Uwe John6, and Rodrigo Torres7,8 5 1 Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. 2 Instituto Milenio de Oceanografía de Chile. 3 UMI 3614, Evolutionary Biology and Ecology of Algae, CNRS-UPMC Sorbonne Universités, PUCCh, UACH. 4 Department of Plant Sciences, University of Oxford, OX1 3RB Oxford, UK. 5 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. 10 6 Marine Biogeosciences | PhytoChange Alfred Wegener Institute – Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany. 7 Centro de Investigación en Ecosistemas de la Patagonia (CIEP), Coyhaique, Chile. 8 Centro de Investigación: Dinámica de Ecosistemas marinos de Altas Latitudes (IDEAL), Punta Arenas, Chile. Correspondence to: Peter von Dassow ([email protected]) 15 Abstract. Marine multicellular organisms inhabiting waters with natural high fluctuations in pH appear more tolerant to acidification than conspecifics occurring in nearby stable waters, suggesting that environments of fluctuating pH hold genetic reservoirs for adaptation of key groups to ocean acidification (OA). The abundant and cosmopolitan calcifying phytoplankton Emiliania huxleyi exhibits a range of morphotypes with varying degrees of coccolith mineralization. We show that E. huxleyi populations in the naturally acidified upwelling waters of the Eastern South Pacific, where pH drops below 20 7.8 as is predicted for the global surface ocean by the year 2100, are dominated by exceptionally overcalcified morphotypes whose distal coccolith shield can be almost solid calcite. -

Ecology of Oceanic Coccolithophores. I. Nutritional Preferences of the Two Stages in the Life Cycle of Coccolithus Braarudii and Calcidiscus Leptoporus
AQUATIC MICROBIAL ECOLOGY Vol. 44: 291–301, 2006 Published October 10 Aquat Microb Ecol Ecology of oceanic coccolithophores. I. Nutritional preferences of the two stages in the life cycle of Coccolithus braarudii and Calcidiscus leptoporus Aude Houdan, Ian Probert, Céline Zatylny, Benoît Véron*, Chantal Billard Laboratoire de Biologie et Biotechnologies Marines, Université de Caen Basse-Normandie, Esplanade de la Paix, 14032 Caen Cedex, France ABSTRACT: Coccolithus braarudii and Calcidiscus leptoporus are 2 coccolithophores (Prymnesio- phyceae: Haptophyta) known to possess a complex heteromorphic life cycle, with alternation between a motile holococcolith-bearing haploid stage and a non-motile heterococcolith-bearing diploid stage. The ecological implications of this type of life cycle in coccolithophores are currently poorly known. The nutritional preferences of each stage of both species, and their growth response to conditions of turbulence were investigated by varying their growth conditions. Of the different cul- ture media tested, only the synthetic seawater medium did not support the growth of both stages of C. braarudii and C. leptoporus. With natural seawater-based media, the growth rate of the haploid phase of both coccolithophores was stimulated by the addition of soil extract (K/2: 0.23 ± 0.02 d–1 and K/2 with soil extract 0.35 ± 0.01 d–1 for the C. braarudii haploid stage), while the diploid phase was not, indicating that the motile stage is capable of utilizing compounds present in soil extract or ingest- ing bacteria that are activated in enriched media. The addition of sodium acetate to the medium also stimulated the haploid phase of C. -

University of Oklahoma
UNIVERSITY OF OKLAHOMA GRADUATE COLLEGE MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE FACULTY in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY By JOSHUA THOMAS COOPER Norman, Oklahoma 2017 MACRONUTRIENTS SHAPE MICROBIAL COMMUNITIES, GENE EXPRESSION AND PROTEIN EVOLUTION A DISSERTATION APPROVED FOR THE DEPARTMENT OF MICROBIOLOGY AND PLANT BIOLOGY BY ______________________________ Dr. Boris Wawrik, Chair ______________________________ Dr. J. Phil Gibson ______________________________ Dr. Anne K. Dunn ______________________________ Dr. John Paul Masly ______________________________ Dr. K. David Hambright ii © Copyright by JOSHUA THOMAS COOPER 2017 All Rights Reserved. iii Acknowledgments I would like to thank my two advisors Dr. Boris Wawrik and Dr. J. Phil Gibson for helping me become a better scientist and better educator. I would also like to thank my committee members Dr. Anne K. Dunn, Dr. K. David Hambright, and Dr. J.P. Masly for providing valuable inputs that lead me to carefully consider my research questions. I would also like to thank Dr. J.P. Masly for the opportunity to coauthor a book chapter on the speciation of diatoms. It is still such a privilege that you believed in me and my crazy diatom ideas to form a concise chapter in addition to learn your style of writing has been a benefit to my professional development. I’m also thankful for my first undergraduate research mentor, Dr. Miriam Steinitz-Kannan, now retired from Northern Kentucky University, who was the first to show the amazing wonders of pond scum. Who knew that studying diatoms and algae as an undergraduate would lead me all the way to a Ph.D. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

An Explanation for the 18O Excess in Noelaerhabdaceae Coccolith Calcite
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 189 (2016) 132–142 www.elsevier.com/locate/gca An explanation for the 18O excess in Noelaerhabdaceae coccolith calcite M. Hermoso a,⇑, F. Minoletti b,c, G. Aloisi d,e, M. Bonifacie f, H.L.O. McClelland a,1, N. Labourdette b,c, P. Renforth g, C. Chaduteau f, R.E.M. Rickaby a a University of Oxford – Department of Earth Sciences, South Parks Road, Oxford OX1 3AN, United Kingdom b Sorbonne Universite´s, UPMC Universite´ Paris 06 – Institut de Sciences de la Terre de Paris (ISTeP), 4 Place Jussieu, 75252 Paris Cedex 05, France c CNRS – UMR 7193 ISTeP, 4 Place Jussieu, 75252 Paris Cedex 05, France d Sorbonne Universite´s, UPMC Universite´ Paris 06 – UMR 7159 LOCEAN, 4 Place Jussieu, 75005 Paris, France e CNRS – UMR 7159 LOCEAN, 4 Place Jussieu, 75005 Paris, France f Institut de Physique du Globe de Paris, Sorbonne Paris Cite´, Universite´ Paris-Diderot, UMR CNRS 7154, 1 rue Jussieu, 75238 Paris Cedex, France g Cardiff University – School of Earth and Ocean Sciences, Parks Place, Cardiff CF10 3AT, United Kingdom Received 10 November 2015; accepted in revised form 11 June 2016; available online 18 June 2016 Abstract Coccoliths have dominated the sedimentary archive in the pelagic environment since the Jurassic. The biominerals pro- duced by the coccolithophores are ideally placed to infer sea surface temperatures from their oxygen isotopic composition, as calcification in this photosynthetic algal group only occurs in the sunlit surface waters. In the present study, we dissect the isotopic mechanisms contributing to the ‘‘vital effect”, which overprints the oceanic temperatures recorded in coccolith calcite. -
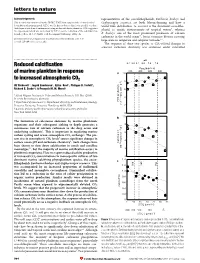
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Coccolithophore Distribution in the Mediterranean Sea and Relate A
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Ocean Sci. Discuss., 11, 613–653, 2014 Open Access www.ocean-sci-discuss.net/11/613/2014/ Ocean Science OSD doi:10.5194/osd-11-613-2014 Discussions © Author(s) 2014. CC Attribution 3.0 License. 11, 613–653, 2014 This discussion paper is/has been under review for the journal Ocean Science (OS). Coccolithophore Please refer to the corresponding final paper in OS if available. distribution in the Mediterranean Sea Is coccolithophore distribution in the A. M. Oviedo et al. Mediterranean Sea related to seawater carbonate chemistry? Title Page Abstract Introduction A. M. Oviedo1, P. Ziveri1,2, M. Álvarez3, and T. Tanhua4 Conclusions References 1Institute of Environmental Science and Technology (ICTA), Universitat Autonoma de Barcelona (UAB), 08193 Bellaterra, Spain Tables Figures 2Earth & Climate Cluster, Department of Earth Sciences, FALW, Vrije Universiteit Amsterdam, FALW, HV1081 Amsterdam, the Netherlands J I 3IEO – Instituto Espanol de Oceanografia, Apd. 130, A Coruna, 15001, Spain 4GEOMAR Helmholtz-Zentrum für Ozeanforschung Kiel, Marine Biogeochemistry, J I Duesternbrooker Weg 20, 24105 Kiel, Germany Back Close Received: 31 December 2013 – Accepted: 15 January 2014 – Published: 20 February 2014 Full Screen / Esc Correspondence to: A. M. Oviedo ([email protected]) Published by Copernicus Publications on behalf of the European Geosciences Union. Printer-friendly Version Interactive Discussion 613 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract OSD The Mediterranean Sea is considered a “hot-spot” for climate change, being char- acterized by oligotrophic to ultra-oligotrophic waters and rapidly changing carbonate 11, 613–653, 2014 chemistry. Coccolithophores are considered a dominant phytoplankton group in these 5 waters. -

Investigation of Cell Growth and Chlorophyll a Content of The
ssing oce & pr B o io i t B e c Hariskos et al., J Bioprocess Biotech 2015, 5:6 f h o n l i a q DOI: 10.4172/2155-9821.1000234 n u r e u s o J Journal of Bioprocessing & Biotechniques ISSN: 2155-9821 Research Article Open Access Investigation of Cell Growth and Chlorophyll a Content of the Coccolithophorid Alga Emiliania huxleyi by Using Simple Bench-Top Flow Cytometry Ioanna Hariskos*, Tobias Rubner and Clemens Posten Institute of Process Engineering in Life Sciences, Karlsruhe Institute of Technology, Germany Abstract The coccolithophorid alga Emiliania huxleyi produces micro-structured calcite particles, which are called coccoliths. Due to their unique and sophisticated structure, coccoliths are highly promising for different industrial applications, such as paper manufacturing, color and lacquer preparation. The mass production of coccoliths requires the evaluation of optimum cultivation conditions. This study investigates the impact of varying irradiance (10-1500 μmol m-² s-1) on growth and chlorophyll a content of two calcifying strains CCMP371 and RCC1216 as well as on the non-calcifying strain RCC1217 (haploid form of RCC1217). The light kinetics contradicts the popular opinion, that E. huxleyi is an extraordinarily light tolerating alga in general. Photoinhibition was already observed at irradiance >500 µmol m-2 s-1 in the case of the calcifying strains. Furthermore, light requirements to grow at maximum growth rate, as well as thresholds towards photoinhibition were considerably different between calcifying and non-calcifying strains. The haplont required significantly higher irradiance to reach maximum -2 -1 µspec (>200 µmol m s ), while being much more tolerant to towards photoinhibition, which occurred not until 800 µmol m-2 s-1. -

Aquatic Species Program Review Proceedings of the March 1985 Principal Investigators Meeting
NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Department of Energy, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, expressed or implied, or assumes any legai liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Printed in the United States of America Available from: National Technical Information Service U.S. Departmenr of Commerce 5285 Port Royal Road Springfield, VA 221 61 Price: Microfiche A01 Printed Copy A1 6 Codes are ~sedfor pricing ail publications. The code is determined by the number of pages in the publication. lnformation pertaining to the pricing codes can be found in the current issue of the following publications, which are generally available in most tibraries; Energy Research Abstracts. (ERA); Government Reports Announcements and Index (GRA and I); Scientific and Technical Abstract Reports (STAR;; and publication. NTIS-PR-360 available from NT1S at the above address. SER IICP-231-2700 UC Category: 61c DE85Ol2137 Aquatic Species Program Review Proceedings of the March 1985 Principal Investigators Meeting 20 - 21 March 1985 Golden, CO June 1985 Prepared under Task No. 4513.10 FTP No. 513 Solar Energy Research Institute A Division of Midwest Research Institute 1617 Cole Boulevard Golden, Colorado 80401 Prepared for the U.S. Department of Energy Contract No. DE-AC02-83CH10093 PREFACE This volume contains progress reports presented by the Aquatic Species Program subcontractors and SERl researchers at the SERl Aquatic Species Review held at SERI, March 20 and 2 1, 1985. -

The Coccolithophore Family Calciosoleniaceae with Report of A
The coccolithophore family Calciosoleniaceae with report of a new species: Calciosolenia subtropicus from the southern Indian Ocean Shramik Patil, Rahul Mohan, Syed Jafar, Sahina Gazi, Pallavi Choudhari, Xavier Crosta To cite this version: Shramik Patil, Rahul Mohan, Syed Jafar, Sahina Gazi, Pallavi Choudhari, et al.. The coccolithophore family Calciosoleniaceae with report of a new species: Calciosolenia subtropicus from the southern Indian Ocean. Micropaleontology, Micropaleontology Press, 2019. hal-02323185 HAL Id: hal-02323185 https://hal.archives-ouvertes.fr/hal-02323185 Submitted on 22 Oct 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The coccolithophore family Calciosoleniaceae with report of a new species: Calciosolenia subtropicus from the southern Indian Ocean Shramik Patil1, Rahul Mohan1, Syed A. Jafar2, Sahina Gazi1, Pallavi Choudhari1 and Xavier Crosta3 1National Centre for Polar and Ocean Research (NCPOR), Headland Sada, Vasco-da-Gama, Goa-403804, India email: [email protected] 2Flat 5-B, Whispering Meadows, Haralur Road, Bangalore-560 102, India 3UMR-CNRS 5805 EPOC, Université de Bordeaux, Allée Geoffroy Saint Hilaire, 33615 Pessac Cedex, France ABSTRACT: The families Calciosoleniaceae, Syracosphaeraceae and Rhabdosphaeraceae belong to the order Syracosphaerales and constitute a significant component of extant coccolithophore species, sharing similar ultrastructural bauplans. -

The Revised Classification of Eukaryotes
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231610049 The Revised Classification of Eukaryotes Article in Journal of Eukaryotic Microbiology · September 2012 DOI: 10.1111/j.1550-7408.2012.00644.x · Source: PubMed CITATIONS READS 961 2,825 25 authors, including: Sina M Adl Alastair Simpson University of Saskatchewan Dalhousie University 118 PUBLICATIONS 8,522 CITATIONS 264 PUBLICATIONS 10,739 CITATIONS SEE PROFILE SEE PROFILE Christopher E Lane David Bass University of Rhode Island Natural History Museum, London 82 PUBLICATIONS 6,233 CITATIONS 464 PUBLICATIONS 7,765 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Biodiversity and ecology of soil taste amoeba View project Predator control of diversity View project All content following this page was uploaded by Smirnov Alexey on 25 October 2017. The user has requested enhancement of the downloaded file. The Journal of Published by the International Society of Eukaryotic Microbiology Protistologists J. Eukaryot. Microbiol., 59(5), 2012 pp. 429–493 © 2012 The Author(s) Journal of Eukaryotic Microbiology © 2012 International Society of Protistologists DOI: 10.1111/j.1550-7408.2012.00644.x The Revised Classification of Eukaryotes SINA M. ADL,a,b ALASTAIR G. B. SIMPSON,b CHRISTOPHER E. LANE,c JULIUS LUKESˇ,d DAVID BASS,e SAMUEL S. BOWSER,f MATTHEW W. BROWN,g FABIEN BURKI,h MICAH DUNTHORN,i VLADIMIR HAMPL,j AARON HEISS,b MONA HOPPENRATH,k ENRIQUE LARA,l LINE LE GALL,m DENIS H. LYNN,n,1 HILARY MCMANUS,o EDWARD A. D.