Effects of Fexofenadine, Diphenhydramine, and Alcohol on Driving Performance a Randomized, Placebo-Controlled Trial in the Iowa Driving Simulator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22-556Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-556Orig1s000 MEDICAL REVIEW(S) MEDICAL OFFICER REVIEW Division Of Pulmonary, Allergy, and Rheumatology Products, HFD-570 APPLICATION: NDA 22-556 TRADE NAME: Karbinal ER™ APPLICANT/SPONSOR: Tris Pharma USAN NAME: Carbinoxamine Extended-Release MEDICAL OFFICER: Peter Starke, MD Oral Suspension TEAM LEADER: Theresa Michele, MD CATEGORY: Antihistamine DATE: February 25, 2013 ROUTE: Oral SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date Submission Date Application/Doc Comments October 4, 2012 October 5, 2012 SD-17 Complete Response submission January 8, 2013 January 9, 2013 SD-20 Response to labeling (formatting) IR RELATED APPLICATIONS Date Application Comments REVIEW SUMMARY: This is clinical review of a Complete Response (CR) to a CR action taken by the Agency on October 7, 2011, for a 505(b)(2) application from Tris Pharma for Carbinoxamine Extended-Release (ER) Oral Suspension, equivalent to 4 mg of carbinoxamine maleate (CM) per 5 mL. The formulation is a sustained release formulation of carbinoxamine maleate suspended in a drug-polistirex resin complex. The proposed Trade Name is Karbinal ER. The application references both the currently available generic immediate-release Carbinoxamine Maleate 4 mg tablets (ANDA 40-442) and oral solution 4 mg/5 mL(ANDA 40-458), marketed under the brand name Palgic and manufactured by Milkart, Inc., and the no-longer-marketed immediate-release innovator products, Clistin 4 mg tablets (NDA 08-915) and 4 mg/5 mL elixir (NDA 08-955), previously marketed by McNeil. McNeil discontinued marketing the Clistin products in the 1990s, and the Orange Book makes the notation that the Clistin products were not discontinued or withdrawn for safety or efficacy reasons. -

Drug and Alcohol Abuse Prevention Handbook FOREWARD
Drug and Alcohol Abuse Prevention Handbook FOREWARD Grayson College recognizes that the illicit use of drugs and/or the abuse of alcohol are a persistent health problem of major proportion affecting our society physically, mentally, and socially. Illicit drug use and /or alcohol abuse can adversely affect an individual’s personal life, safety, health, and mental and physical performance. It is the intent of GC to provide employees and students pertinent information related to illicit drug use and/or alcohol abuse in an effort to prevent such harm. GC is committed to promoting and maintaining a work and academic environment that is free from illegal alcohol and drug use and abuse, in accordance with all federal, state, and local laws. Students, employees, and visitors are prohibited from possessing, consuming, manufacturing, dispensing, or being under the influence of alcohol/illegal drugs or engaging in improper self- medication while on college property or college business. Any member of the college community who violates this policy is subject to both prosecution and punishment under federal, state, and local laws to disciplinary proceedings by the college. This alcohol/drug policy is not designed to punish people for seeking rehabilitation. All information about those individuals who voluntarily avail themselves of drug or alcohol counseling or rehabilitation will not be used as a basis for disciplinary action or be used against an individual in any way. College employees and students who violate the alcohol/drug policy shall be informed about and referred to services to assist them in determining whether they are abusing drugs and alcohol or are chemically dependent. -

For Personal Use Only
SedatiWITH ANTIi ® Dowden Health Media CopyrightFor personal use only Initiate the antipsychotic at a reasonable, not overly high dose, then use a nonantipsychotic to help control insomnia, anxiety, and agitation For mass reproduction, content licensing and permissions contact Dowden Health Media. pSYCHIATRY i PSYCHOTICSon edation is a frequent side effect of antipsychot- ics, especially at relatively high doses. Antipsy- S chotics’ sedative effects can reduce agitation in acute psychosis and promote sleep in insomnia, but Manage, don’t long-term sedation may: • interfere with schizophrenia patients’ efforts to go accept adverse to work or school or engage in normal socialization • prevent improvement from psychosocial training, psychiatric rehabilitation, and other treatments. ‘calming’ eff ect This article discusses how to manage acute psycho- sis without oversedation and ways to address persistent sedation and chronic insomnia with less-sedating anti- Del D. Miller, PharmD, MD Professor of psychiatry psychotics or adjunctive medications. University of Iowa Carver College of Medicine Iowa City Neurobiology or psychopharmacology? Many patients experience only mild, transient som- nolence at the beginning of antipsychotic treatment, and most develop some tolerance to the sedating ef- fects with continued administration. Others may have persistent daytime sedation that interferes with nor- mal functioning. Sedation is especially common in elderly patients re- ceiving antipsychotics. Compared with younger patients, older patients receiving -

The Case of Ketamine Allergy
CASE REPORT The Case of Ketamine Allergy William Bylund, MD Department of Emergency Medicine, Naval Medical Center Portsmouth, Portsmouth, Virginia Liam Delahanty, MD Maxwell Cooper, MD Section Editor: Rick A. McPheeters, DO Submission history: Submitted April 3, 2017; Revision received June 29, 2017; Accepted July 11, 2017 Electronically published October 3, 2017 Full text available through open access at http://escholarship.org/uc/uciem_cpcem DOI: 10.5811/cpcem.2017.7.34405 Ketamine is often used for pediatric procedural sedation due to low rates of complications, with allergic reactions being rare. Immediately following intramuscular (IM) ketamine administration, a three-year-old female rapidly developed facial edema and diffuse urticarial rash, with associated wheezing and oxygen desaturation. Symptoms resolved following treatment with epinephrine, dexamethasone and diphenhydramine. This case presents a clinical reaction to ketamine consistent with anaphylaxis due to histamine release, but it is uncertain whether this was immunoglobulin E mediated. This is the only case reported to date of allergic reaction to IM ketamine, without co- administration of other agents. [Clin Pract Cases Emerg Med.2017;1(4):323–325.] INTRODUCTION cardiac monitor, end tidal CO2 (ETCO2) and pulse oximetry Ketamine is a common medication, used in isolation as well (POx), in addition to being placed on two liters nasal cannula as with other agents, for pediatric sedation in the emergency (NC). No intravenous (IV) access was obtained prior to sedation. department (ED). It is often turned to because of its efficacy, ease Seventy milligrams (4.4mg/kg) of ketamine was administered IM of use, and favorable safety profile. Common side effects of into the right thigh. -

FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine Hydrochloride
ID1089 MRP _ UK Version: 07 Review Date: 19/02/2020 PACKAGE LEAFLET: INFORMATION FOR THE USER FEXOFENADINE HYDROCHLORIDE 120 MG FILM-COATED TABLETS FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, (see section 4). In this leaflet: 1. What Fexofenadine hydrochloride is and what it is used for 2. What you need to know before you take Fexofenadine hydrochloride 3. How to take Fexofenadine hydrochloride 4. Possible side effects of Fexofenadine hydrochloride 5. How to store Fexofenadine hydrochloride 6. Contents of the pack and other information 1. WHAT FEXOFENADINE HYDROCHLORIDE IS AND WHAT IT IS USED FOR FEXOFENADINE HYDROCHLORIDE Contains fexofenadine hydrochloride which is an antihistamine. Only Fexofenadine hydrochloride 120 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with hay fever (seasonal allergic rhinitis) such as sneezing, itchy, running or blocked nose and itchy, red and watery eye). Fexofenadine hydrochloride 180 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with long term allergic skin reactions( chronic idiopathic urticaria) such as itching, swelling and rashes 2. -

Diphenhydramine Dosage Sheet Concord Pediatrics, P.A
Diphenhydramine Dosage Sheet Concord Pediatrics, P.A. (603) 224-1929 BRAND NAMES: Benadryl INDICATIONS: Treatment of allergic reactions, nasal allergies, hives and itching. FREQUENCY: Repeat every 6 hours as needed. Don't give more than 4 times a day. DOSAGE: Determine by using the table below. Please speak with a medical provider before giving Diphenhydramine to a child under 1 year old. Child’s Dose in mg Children’s Liquid Chewable/Fastmelts Tabs/Caps/Gels Weight (lb) (12.5mg/ 5mL) 12.5mg tablets 25mg tablets *12-16 lbs 6.25 mg 2.5 mL *17-19 lbs 9.375 mg 3.75 mL *20-24 lbs 10 mg 4 mL 25-37 lbs 12.5 mg 5 mL 1 tab 38-49 lbs 18.75 mg 7.5 mL 1.5 tabs 50-69 lbs 25 mg 10 mL 2 tabs 1 tab 70-99 lbs 37.5 mg 15 mL 3 tabs 1.5 tabs >100 lbs 50 mg 20 mL 4 tabs 2 tabs Table Notes: *AGE LIMIT: For allergies, don't use under 1 year of age (Reason: it's a sedative). For colds, not recommended at any age (Reason: no proven benefits) and should be avoided if under 4 years old. Avoid multi-ingredient products in children under 6 years of age (Reason: FDA recommendations 10/2008). MEASURING the DOSAGE: Syringes and droppers are more accurate than teaspoons. If possible, use the syringe or dropper that comes with the medicine. If not, medicine syringes are available at pharmacies. Regular spoons are not reliable. ADULT DOSAGE: 50 mg Why use Diphenhydramine? Antihistamines can be used to treat your child’s runny nose, itchy eyes, and sneezing due to allergies. -

Kiwiherb Valerian Oral Liquid Valerian Root Extract
PATIENT INFORMATION LEAFLET: stress and to aid sleep, exclusively based on long standing use. 2. Before you take this product DO NOT TAKE this product if you are: • Allergic to Valerian or any other ingredients in this product (see section 6) • Under 18 years of age • Pregnant or breast feeding • Taking medicines known to interact with alcohol (e.g. metronidazole) • Already taking a medicine for sleep or Kiwiherb Valerian Oral Liquid anxiety Valerian Root Extract Taking other medicines Tell your healthcare professional, e.g. a doctor or pharmacist, if you are taking any other medicines Read all of this leaflet carefully because it contains including herbal medicines or medicines that did important information for you. not require a prescription. This medicine is available without prescription. Driving and using machines However you still need to use Kiwiherb Valerian carefully to get the best results from it. This product may cause drowsiness. If affected, do not drive or operate machinery Keep this leaflet. You may want to read it again. Take Special Care with this product Ask your healthcare professional, e.g. a doctor or pharmacist, if you need more information or This product contains alcohol (45% ethanol), i.e. advice. up to 2.25 ml per 5 ml dose, (equivalent to 45 ml beer or 18.8 ml wine), and may be harmful to If the condition worsens or symptoms do not those suffering from alcoholism. improve after 2 weeks, a qualified healthcare professional, e.g. a doctor or pharmacist, should be To be taken into account in pregnant or consulted. -

Neurological Complications of Nitrous Oxide Abuse
Katherine Shoults, MD Case report: Neurological complications of nitrous oxide abuse A patient who presented with limb paresthesia and B12 deficiency was eventually diagnosed with subacute combined degeneration neuropathy secondary to nitrous oxide abuse. Case data ABSTRACT: A 34-year-old female ary to nitrous oxide (“laughing gas”) A 34-year-old female presented with with a history of alcohol and crystal abuse that had affected B12 activa- a 2-week history of progressive bilat- methamphetamine abuse presented tion. The patient was continued on eral limb paresthesia and tenderness, to the emergency department with B12 therapy, neurology follow-up as well as an inability to balance. She limb paresthesia and difficulty walk- was arranged, and addiction coun- had been well previously, although ing. At a primary care visit a week seling services were recommended. she did have a history of alcohol and earlier her progressive neurological Unfortunately, the patient was lost crystal methamphetamine abuse. She compromise had been viewed in the to follow-up after discharge from the had abstained from crystal metham- context of anemia and she was start- hospital. Physicians should be aware phetamine for 5 years and from alco- ed on daily B12 injections. Further that nitrous oxide is easy to acquire hol for 2 months. She was working as a investigations in hospital revealed in the form of commercially available care aid and denied using illegal drugs diminished proprioception, hyperal- cartridges or whipped cream canis- currently, but reported she had been gesia with pinprick and temperature ters called “whippits” and abuse of inhaling nitrous oxide (“laughing tests, a wide-based high-steppage nitrous oxide is increasingly com- gas”) for 6 months, with an escalation gait, elevated levels of B12 and ho- mon. -

SAD.002 Alcohol and Drugs
ALCOHOL AND DRUG POLICY Southern Oregon University is committed to promoting an environment that supports the health and well-being of every member of the campus community. Since drug and alcohol abuse can seriously impair an individual’s personal and academic functioning, the University helps campus members make responsible decisions about drugs and alcohol. It is Southern’s obligation, therefore, to provide pertinent drug and alcohol information, educational opportunities, prevention-related activities, individual support and referral services, and enforcement of University rules regarding the use of alcohol and illegal drugs. In keeping with this policy and the intent of Public Law 101-226, Section 22: Drug-Free Schools and Campuses, it is our obligation and responsibility to inform you of the health risks associated with the use of various illicit drugs, nicotine, and the abuse of alcohol. Please note that any substance used through needle-sharing increases the risk of contracting AIDS and hepatitis B. Controlled Substances: Type of Drug and Possible Health Risks 1. Stimulants – speed up action of central nervous system • Amphetamines (speed). Hallucinations; heart problems; malnutrition; dependency; paranoid psychosis; death. Affects fetal development. • Cocaine (coke, crack) — Classified as a narcotic. Confusion; depression; convulsions; damaged nasal membranes; lung lesions; dependency; coma; paranoid psychosis; death. Affects fetal development. • MDMA (ecstasy). Short-term: euphoria; dehydration; loss of inhibition. Long-term: danger to cognitive learning and memory impairment. 2. Depressants – relax central nervous system • Barbiturates (downers). Tranquilizers and methaqualone (ludes). Confusion; loss of coordination; tolerance; dependency; seizures; coma; death. • Especially dangerous in combination with alcohol. 3. Cannabis – alters perception and mood • Marijuana and hashish. -

Alcohol Hangover Headache
Headache ISSN 0017-8748 C 2007 the Authors doi: 10.1111/j.1526-4610.2006.00694.x Journal compilation C 2007 American Headache Society Published by Blackwell Publishing Expert Opinion Alcohol Hangover Headache Case History submitted by Randolph W. Evans, MD Expert opinion submitted by Christina Sun, MD; Christine Lay, MD Key words: alcohol hangover headache, migraine (Headache 2007;47:277-279) In his 1954 first novel, “Lucky Jim,” Sir Kingsley she has no ill effects. She is healthy with no history of Amis describes the delayed effects of drinking port significant headaches. on the titular history lecturer upon awakening in the morning. “Dixon was alive again. Consciousness was QUESTIONS upon him before he could get out of the way; not for What is the prevalence and cause of alcohol hang- him the slow, gracious wandering from the halls of over headache (AHH)? What are the latency, features, sleep, but a summary, forcible ejection. ... The light and duration of the headache? Is the risk of develop- did him harm, but not as much as looking at things ment of AHH related to the type or amount of alcohol did; he resolved, having done it once, never to move consumed? How can you distinguish between AHH his eyeballs again. A dusty thudding in his head made and migraine triggered by alcohol? Are there any ef- the scene before him beat like a pulse. ...he sat up a fective interventions or treatments for AHH? little, and what met his bursting eyes roused to a frenzy the timpanist in his head.” EXPERT COMMENTARY Alcohol hangover, or “veisalgia,” is a well-known CASE and common phenomenon that generally occurs af- A few hours after drinking 3 glasses of any type ter heavy consumption of alcohol. -

Problematic Use of Nitrous Oxide by Young Moroccan–Dutch Adults
International Journal of Environmental Research and Public Health Article Problematic Use of Nitrous Oxide by Young Moroccan–Dutch Adults Ton Nabben 1, Jelmer Weijs 2 and Jan van Amsterdam 3,* 1 Urban Governance & Social Innovation, Amsterdam University of Applied Sciences, P.O. Box 2171, 1000 CD Amsterdam, The Netherlands; [email protected] 2 Jellinek, Department High Care Detox, Vlaardingenlaan 5, 1059 GL Amsterdam, The Netherlands; [email protected] 3 Amsterdam University Medical Center, Department of Psychiatry, University of Amsterdam, P.O. Box 22660, 1100 DD Amsterdam, The Netherlands * Correspondence: [email protected] Abstract: The recreational use of nitrous oxide (N2O; laughing gas) has largely expanded in recent years. Although incidental use of nitrous oxide hardly causes any health damage, problematic or heavy use of nitrous oxide can lead to serious adverse effects. Amsterdam care centres noticed that Moroccan–Dutch young adults reported neurological symptoms, including severe paralysis, as a result of problematic nitrous oxide use. In this qualitative exploratory study, thirteen young adult Moroccan–Dutch excessive nitrous oxide users were interviewed. The determinants of problematic nitrous oxide use in this ethnic group are discussed, including their low treatment demand with respect to nitrous oxide abuse related medical–psychological problems. Motives for using nitrous oxide are to relieve boredom, to seek out relaxation with friends and to suppress psychosocial stress and negative thoughts. Other motives are depression, discrimination and conflict with friends Citation: Nabben, T.; Weijs, J.; van or parents. The taboo culture surrounding substance use—mistrust, shame and macho culture— Amsterdam, J. Problematic Use of frustrates timely medical/psychological treatment of Moroccan–Dutch problematic nitrous oxide Nitrous Oxide by Young users. -
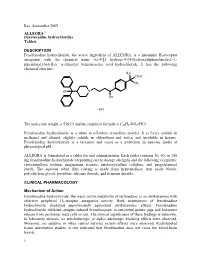
Fexofenadine Hydrochloride) Tablets
Rev. September 2005 ALLEGRA® (fexofenadine hydrochloride) Tablets DESCRIPTION Fexofenadine hydrochloride, the active ingredient of ALLEGRA, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure: The molecular weight is 538.13 and the empirical formula is C32H39NO4•HCl. Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH. ALLEGRA is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hypromellose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine