Prescription Drug Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The People Shaping the Industry
DRSN041208p81 4/21/08 3:46 PM Page 81 ANNUALANNUAL TheREPORT people shaping the industry --------------------------------2 Chains expand focus amid uncertain economy -------------15 The Drug Store News Power Rx 50---------------------------16 Mass retailers push pharmacy by expanding programs ---17 Regional players strengthen foothold by finding niche-----18 Supermarkets emphasize health/nutrition connection------19 Drug StoreStore News News www.drugstorenews.comwww.drugstorenews.com 08AprilApril 21, 21, 2008 2008• •81 1 DRSN_042108_p83.qxd 4/9/08 8:47 PM Page 83 08 ANNUALREPORT Bill Baxley, Kerr Drug ill Baxley, senior vice Baxley has been with Kerr for crease inventory.” The big- president of merchandis- more than 40 years, beginning gest long-term challenge? B ing and marketing for as a stock clerk in 1967. After “Constantly reinventing Kerr Kerr Drug, said if he weren’t in graduating from pharmacy Drugs,” he said. the drug store industry, he’d be school in 1971, he began work- Baxley said the most sur- of service to humanity, “devel- ing at Kerr’s Store No. 1 in prising development of the oping goals for peace.” Raleigh, N.C. past year occurred outside Those who know Baxley As a retailer, Baxley said with the “collapse of the finan- probably wouldn’t be surprised his top goal this year is to cial markets,” and of Bear by his altruistic leanings. “improve margin and de- Stearns in particular. Paul Beahm, Wal-Mart aul Beahm, senior vice the healthcare arena, where he’s tinue today, but after a major president and general spent the majority -
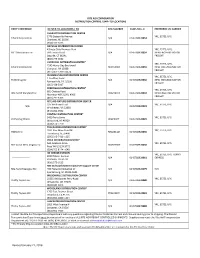
Revised January 21, 2016
RITE AID CORPORATION DISTRIBUTION CENTERS / SHIP–TO-LOCATIONS ENTITY REFERENCE DC SHIP-TO-LOCATIONS / PO DEA NUMBER DUNS NO.+ 4 PREFERRED LTL CARRIER CHARLOTTE DISTRIBUTION CENTER 1776 Statesville Avenue YRC, ESTES, UPS Eckerd Corporation N/A 0145788920053 Charlotte, NC 28206 (704) 371-3653 DAYVILLE DISTRIBUTION CENTER Killingly Oaks Business Park YRC, ESTES, UPS, PJC Distributions Inc 500 Forbes Road N/A 0145788920054 NEW ENGLAND MOTOR Dayville, CT 06241 FREIGHT (860) 779-0632 LIVERPOOL DISTRIBUTION CENTER* YRC, ESTES, UPS, 7245 Henry Clay Boulevard Eckerd Corporation RE0356003 0145788920055 NEW ENGLAND MOTOR Liverpool, NY 13088 FREIGHT (315) 451-5700 x2274 PHILADELPHIA DISTRIBUTION CENTER YRC, ESTES, UPS, 1 Geoffrey Road Thrift Drug Inc N/A 0145788920056 NEW ENGLAND MOTOR Fairless Hills, PA 19030 FREIGHT (215) 428-5917 PERRYMAN DISTRIBUTION CENTER* YRC, ESTES, UPS, 601 Chelsea Road Rite Aid of Maryland Inc RR0236073 0145788920010 NEW ENGLAND MOTOR Aberdeen MD 21001-4306 FREIGHT (410) 297-6363 RITE AID FIXTURE DISTRIBUTION CENTER 325 Welltown Road N/A YRC, ESTES, UPS N/A 0145788920023 Winchester, VA 22603 (540) 662-3552 PONTIAC DISTRIBUTION CENTER* 5400 Perry Drive YRC, ESTES, UPS Perry Drug Stores 002230PIY 0145788920029 Waterford, MI 48329 (248) 674-7770 TUSCALOOSA DISTIBUTION CENTER* 3931 Rice Mine Road NE YRC, ESTES, UPS HARCO Inc RH0231124 0145788920035 Tuscaloosa, AL 35406 (205) 345-7419 x225 POCA DISTRIBUTION CENTER* 160 Jacobson Drive YRC, ESTES, UPS Rite Aid of West Virginia Inc 004569RDY 0145788920050 Poca WV 25159-9772 (304) -

Revised December 6, 2018
RITE AID CORPORATION DISTRIBUTION CENTERS / SHIP–TO-LOCATIONS ENTITY REFERENCE DC SHIP-TO-LOCATIONS / PO DEA NUMBER DUNS NO.+ 4 PREFERRED LTL CARRIER LIVERPOOL DISTRIBUTION CENTER* 7245 Henry Clay Boulevard YRC, NEMF, FEDEX, ESTES, Eckerd Corporation RE0356003 0145788920055 Liverpool, NY 13088 FREIGHT, OLD DOMINION (315) 451-5700 x2274 PHILADELPHIA DISTRIBUTION CENTER 1 Geoffrey Road YRC, NEMF, FEDEX, ESTES, , Thrift Drug Inc N/A 0145788920056 Fairless Hills, PA 19030 OLD DOMINION (215) 428-5917 PERRYMAN DISTRIBUTION CENTER* 601 Chelsea Road YRC, NEMF, FEDEX, ESTES, Rite Aid of Maryland Inc RR0236073 0145788920010 Aberdeen MD 21001-4306 OLD DOMINION, SAIA (410) 297-6363 RITE AID FIXTURE DISTRIBUTION CENTER YRC, FEDEX, ESTES, 325 Welltown Road N/A N/A 0145788920023 OLD DOMINION Winchester, VA 22603 (540) 662-3552 PONTIAC DISTRIBUTION CENTER* YRC, FEDEX, ESTES, OLD 5400 Perry Drive Perry Drug Stores 002230PIY 0145788920029 DOMINION, SAIA Waterford, MI 48329 (248) 674-7770 ICE CREAM DIVISION YRC, FEDEX, ESTES, OLD 9200 Telstar Avenue ---------------------- N/A 0145788920061 DOMINION, SAIA El Monte, CA 91731 (626) 571-0122 WILSONVILLE DISTRIBUTION CENTER YRC, FEDEX, ESTES, OLD 29555 SW Boones Ferry Road Thrifty Payless Inc N/A 0145788920080 DOMINION, SAIA Wilsonville, OR 97070 (503) 685-6013 WOODLAND DISTRIBUTION CENTER* 1755 East Beamer Street YRC, FEDEX, ESTES, OLD Thrifty Payless INC RT0223874 0145788920081 Woodland, CA 95776 DOMINION, SAIA (530) 661-1800 x124 LANCASTER DISTRIBUTION CENTER YRC, FEDEX, ESTES, OLD 2801 West Avenue H Thrifty Payless INC N/A 0145788920088 DOMINION, SAIA Lancaster, CA 93536 (661) 951-7565 Contact the Rite Aid Transportation Department with any questions regarding Rite Aid Preferred Carriers and inbound routing prior to shipping. -

Robert J. Desalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944
Robert J. DeSalvo Papers Business Combinations in the Cosmetic and Pharmaceutical Industries 1944 - 1990 Collection #107 Abstract Robert J. DeSalvo’s research focused on business combinations (acquisitions, mergers, and joint ventures in the cosmetic and pharmaceutical industries. This topic was the basis for his master’s thesis in pharmacy administration at the University of Pittsburgh and continued as a life-long interest. This collection consists of two series of notebooks that Dr. DeSalvo developed to record relevant business combinations. The first series records acquisitions, proposed acquisitions, mergers, and joint ventures for the period of 1944 –1990 in an alphabetical arrangement. The information on these entries is cumulative so that the history of an organization is collected in one place. The second series of notebooks is arranged in chronological blocks. The information is arranged alphabetically by the name of the acquirer. The name of the acquired (merged), type of combination (acquisition, proposed acquisition, joint venture) and the date is also provided. The information is cross-referenced between the two series so that the researcher can approach the information by the name of the parent company or chronologically. Dr. DeSalvo used this resource for many of his publications as well as his master’s thesis. A copy of these publications and his thesis make up the remainder of the collection. Donor Gift of Barbara DeSalvo, 2000 Biography Robert James DeSalvo was born on July 20, 1933 in Toledo, OH. He died on January 23, 1993 in Cincinnati, OH. DeSalvo graduated from high school in Toledo and attended pharmacy school at the University of Toledo where he received his B.S. -

鉴美国经验研判国内零售药房行业整合趋势 强烈推荐 2018.03.06 医药生物行业 唐爱金(分析师) 冯俊曦(研究助理) 电话: 020-88836115 020-88836115 行业指数走势 邮箱: [email protected] [email protected]
证券研究报告 零售药房专题报告 鉴美国经验研判国内零售药房行业整合趋势 强烈推荐 2018.03.06 医药生物行业 唐爱金(分析师) 冯俊曦(研究助理) 电话: 020-88836115 020-88836115 行业指数走势 邮箱: [email protected] [email protected] 执业编号: A1310516100001 沪深300 医药生物指数 美国行业端经验:行业整合“先单体后连锁”且持续稳定,龙头显著受益 20% 美国早在 18 实际已实现医药分离,行业趋势更多偏重于集中度提升(CR3 约 15% 85.3%)。与医药分离相比,其整合经验更值得我们学习。 10% 5% 政策扶持连锁药房、连锁药房集采价格优势、药师人才充足等因素推动美国零售药 0% 房行业集中度提升,整合过程当中体现出“先整合单体药房后中小连锁”、“整合步 17/8 17/9 17/10 17/11 17/12 18/1 18/2 -5% 伐持续稳定”等特征。三巨头顺势整合扩张纽约州、德州、加州等高潜力市场,业 -10% 绩成长持续高于行业,龙头效应显现(1993 年-2015 年 CVS、Walgreens、Rite Aid 销售年复合增长分别为 17.15%、13.90%、9.75%,远高于行业 5.58%年复合增长)。 股价表现 美国企业端经验:优秀企业需具备规模优势和利润空间,二级市场持续享 涨跌(%) 1M 3M 6M 受估值溢价 美国零售药房企业端经验主要有两点:( 1)优秀整合型零售药房需具备“迅速提升 绝对表现 -7.86 -7.90 0.00 规模优势”、“保持充足利润空间”两大属性。CVS 作为全美第一大药房,其两大属 相对表现 -1.31 -3.76 -7.89 性均优于 Walgreens 和 Rite Aid。在规模优势方面,CVS 总营收、单店营收规模 行业估值走势 1777.75 亿美元均高于 Walgreens1457.05 亿美元和 Rite Aid673.90 亿美元;在利润空 间方面,CVS 能常年持续保持近 3.39%净利润率(与 Walgreens 的 3.45%无明显差 医药生物估值 异),高于 Rite Aid 的-0.33%;( 2)CVS、Walgreens 由于长期持续受益于行业整合 80 趋势且竞争优势逐步扩大,其二级市场估值溢价明显,2007-2016 年 CVS、Walgreens 60 的 PEG 分别为 2.31 和 2.33。鉴于国内四大上市公司行业受益明显且竞争优势持续 40 扩大,理应获得估值溢价(详细数据见正文) 20 美国零售药房发展经验与行业趋势在国内的借鉴与验证: 0 对国内的经验借鉴与趋势验证主要有两点:(1)与美国整合次序类似,国内整合体 现“先单体后中小连锁”的趋势,中国药店连锁化率从 2012 年的 36.02%提升至 2016 年的 49.40%,百强药店市占率仅 29.10%,我们认为未来行业整合机会侧重于中小 行业估值 连锁;(2)通过对中美药房运营成本测算,我们认为共性是龙头企业的规模和竞争 当期估值 43.03 优势逐步扩大(美国大型连锁通过成本优势逼迫中小连锁/单体药房退出一线地段, 平均估值 38.12 国内上市药房与三板药房利润空间逐步拉大), 国内龙头药房增速持续高于行业 历史最高 74.37 (13-16 年上市公司销售复合增长均值为 21.77%,高于行业均值 9.52%),四家上 历史最低 18.80 市零售药房 38.27%-39.85%的平均毛利率、5.62%-6.37%之间的平均净利率高于行 业均值。中小型药房净利率空间不断下降,其盈利能力与上市公司不断拉大。 相关报告 精选投资标的 广证恒生-大参林(603233)-公司深度-稳健扩张 大参林(603233):以华南为根基(广东门店超过 1809 家),成功开拓河南、广西 的华南零售龙头,多维度精细化运营造就出色盈 等潜力市场; 利能力-2017.12.17 一心堂(002727):核心区域密集布点,云南、海南、广西实现盈利,川渝等预计 逐步改善; 老百姓(603883):门店分布全国超过 16 个省市,跨域区整合效果出色,未来发展 空间较大; 益丰药房(603939):布局湖南、湖北、上海等七个省市,以精细化管理实现跨区 域稳定经营 风险提示:政策落地、整合程度、处方外流不达预期、跨省经营风险、行 业增速下行风险 零售药房专题报告 目录 图表目录 .......................................................................................................................................................... -

Net-Leased Rite Aid for Sale 600 St
OFFERING MEMORANDUM Net-Leased Rite Aid for Sale 600 St. James Avenue, Goose Creek, SC 29445 (Charleston SC MSA) CONFIDENTIAL INFORMATION INFORMATION NOT WARRANTED This Offering Memorandum and any subsequent THE OFFERING MEMORANDUM SHALL NOT BE DEEMED evaluation material you may be provided (collectively TO REPRESENT THAT STATE OF AFFAIRS OF THE PROPERTY known as the “Offering Memorandum”) is intended OR CONSTITUTE AN INDICATION THAT THERE HAS BEEN solely for your limited use in considering whether to NO CHANGE IN THE BUSINESS OR AFFAIRS OF THE pursue negotiations to acquire 600 St. James Avenue (the PROPERTY SINCE THE DATE OF PREPARATION OF THE “Property”) located in Goose Creek, South Carolina. The OFFERING MEMORANDUM. Property is being marketed for sale by Ackerman & Co. (“Broker”). The information provided in the Offering Memorandum has been gathered from sources that are deemed reliable, The Offering Memorandum contains brief, selected but the Broker does not warrant or represent that the information pertaining to the business and affairs of the information is true or correct. Prospective offerors are Property and has been prepared by Broker. It does not, advised to verify information independently. The Offering however, purport to be all-inclusive or to contain all of Memorandum is not to be construed as an offer or as any the information that a prospective purchaser may desire. part of a contract to sell the property. Broker makes no representation or warranty, express or implied, as to the accuracy or completeness of the Offering Furthermore, the inclusion or exclusion in the Offering Memorandum or any of its contents, and no legal liability Memorandum of information relating to asbestos or is assumed to be implied with respect thereto. -

Wpsmemberguide (1).Pdf
The following paragraph applies when your application for insurance is included in your Member Guide. IMPORTANT NOTICE CONCERNING STATEMENTS IN THE APPLICATION FOR YOUR INSURANCE Please read the copy of the application attached to your policy that's contained in this Member Guide. Omissions or misstatements in the application could cause an otherwise valid claim to be denied. Carefully check the application and write to WPS Underwriting Department within 10 days if any information shown on the application is not correct and complete or if any requested medical history has not been included. Our address is: Health Underwriting, WPS Health Insurance, P.O. Box 7898, Madison WI 53707-7898. The application is part of the insurance policy. The insurance coverage was issued on the basis that the answers to all questions and any other material information shown on the application are correct and complete. 12449-051-0604 Please also see the provision called "Copy of Your Individual Policy Application" at the bottom of the second page of this Member Guide and the copy of your application for your insurance that's the last document contained in this Member Guide. P12035001\ISSUE 000000\0381\DBIC450\88\174388 \00004\02\000647418 Welcome to WPS! We're pleased you've chosen WPS to take care of your benefits needs. As one of our valued customers, you can be assured top-notch benefits and service. As you come to know us, you'll find a philosophy of service that lies at the heart of all our operations. Our employees understand the importance of sound coverage and share a keen understanding of our customers' service expectations. -

1991 December
MICHIGAN JEWISH HISTORY JEWISH HISTORICAL SOCIETY OF MICHIGAN Reminiscences of Fred Butzel, NCJW Centennial, Slomovitz by Rockaway, Complete Index Volumes 1-31. Volume 32 Winter 1991, Kislev 5752 MICHIGAN JEWISH HISTORY (cc:n ywin4) onner i nnc op43n il`vr nve .. When your children shall ask their parents in time to come... Joshua 4:21 Volume 32 Winter 1991 - Kislev 5752 Reminiscences of Fred Butzel: 1877-1948 2 as told to William Boxerman, 1941 In Memoriam: Morris Friedman: 1990-1991 12 Philip Slomovitz: Jewish Journalist 13 Robert Rockaway Update: 1991 16 Philip Slomovitz 100 Years of Service: National Council of Jewish Women, Greater Detroit Section Dorothy Kaufman 18 Early Recollections: YWHA 24 Lea Damsky Field Index of Michigan Jewish History, 1959-1991 26 Book Review: United States Jewry, by Jacob Rader Marcus 41 reviewed by Dr. Bernard Goldman Book Review: Harmony and Dissonance: Voices of Jewish 42 Identity in Detroit, 1914-1966, by Sidney Bolkosky reviewed by Bernard Wax Tributes 44 Footnotes from the Editor 45 Presidents Message 1991 46 Calendar of Future Events 47 Officers and Board 48 EDITOR Judith Levin Cantor EDITORIAL ADVISOR Leonard N. Simons MICHIGAN JEWISH HISTORY is published by The Jewish Historical Society of Michigan. Correspondence concerning editorial matters should be sent to the Editor, J.H.S. 6600 W. Maple Rd. W. Bloomfield, MI 48322. The Society assumes no responsibility for statements made by contributors. MICHIGAN JEWISH HISTORY is available on microfilm from University Microfilms International, 300 N. Zeeb Road, Ann Arbor, MI 48106. Articles in this journal are indexed in Historical Abstracts and in America: History & Life. -

Rite Aid Corporation & Affiliates Legal Ownership Structure As of 3/1/10
Rite Aid Corporation & Affiliates Legal Ownership Structure As of 3/1/10 Rite Aid Corporation DE 4/15/68 23-1614034 001-00031 Rite Aid of Michigan, Inc. Thrifty PayLess, Inc. K & B, Incorporated 537 Elm Street Corporation Fiona One Corp. JCG (PJC) USA, LLC Rite Aid of Connecticut, Inc. Rite Aid of New Jersey, Inc. Rite Aid of Vermont, Inc Rite Aid of Ohio, Inc. Rite Aid Hdqtrs. Corp. RI 4/4/96 CA 5/14/92 DE 2/19/98 MI 6/19/84 DE 10/29/92 23-2962033 DE 8/18/06 CT 12/19/74 NJ 10/29/71 VT 1/20/75 OH 10/12/71 DE 8/27/84 25-1865457 38-0857390 95-4391249 51-0346254 Store #1713 26-0169455 23-1940645 23-1940648 23-1940942 23-1940651 23-2308342 (Inactive) 073-23225 623/650 660/008/009/015 017-47100 Surplus #37882 (Sold 2/25/99) 607 631 646 636 002-00030 , 09501-09999 Acquired 12/12/96 Acquired 8/27/97 657-659 Broad St. Corp. GDF, Inc. 1740 Associates, LLC NJ 4/11/91 MD 1/23/81 MI 6/24/98 The Jean Coutu Group (PJC) Rite Aid of Delaware, Inc. 764 South Broadway-Geneva, Ohio, LLC 80-0052338 34-1343867 39/41 Hightstown Road, LLC Store # 1740 Thrifty Corporation OH 3/9/98 Agentrics LLC 631-00511 Prop sold 1/8/99 (Inactive) USA, Inc. DE 10/6/71 NJ 9/14/05 Rite Aid of Virginia, Inc. CA 12/14/35 K & B Alabama Corporation 23-1974076 95-1297550 (formerly WWRE – LLC) DE 8/6/86 23-1940646 VA 10/22/73 Store # 1167 Relo AL 6/29/84 015-40020,40030,40040,40050,40060, 04-2925810 608 23-1940653 41300 ( Must Keep for Tax Purposes) 72-1011085 52-2242825 3581 Carter Hill Road - 073-23200 647/012 662 .108% Interest Montgomery Corp. -

United States Securities and Exchange Commission Form
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Fiscal Year Ended February 27, 2021 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Transition Period From To Commission File Number 1-5742 RITE AID CORPORATION (Exact name of registrant as specified in its charter) Delaware 23-1614034 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 30 Hunter Lane, Camp Hill, Pennsylvania 17011 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (717) 761-2633 Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $1.00 par value RAD New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to section 13 or section 15(d) of the Exchange Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -
United States Securities and Exchange Commission Form
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Fiscal Year Ended March 4, 2017 OR អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Transition Period From To Commission File Number 1-5742 RITE AID CORPORATION (Exact name of registrant as specified in its charter) Delaware 23-1614034 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 30 Hunter Lane, Camp Hill, Pennsylvania 17011 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (717) 761-2633 Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Common Stock, $1.00 par value New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ፤ No អ Indicate by check mark if the registrant is not required to file reports pursuant to section 13 or section 15(d) of the Exchange Act. Yes អ No ፤ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

United States Securities and Exchange Commission Form
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Fiscal Year Ended March 2, 2013 OR អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For The Transition Period From To Commission File Number 1-5742 RITE AID CORPORATION (Exact name of registrant as specified in its charter) Delaware 23-1614034 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 30 Hunter Lane, Camp Hill, Pennsylvania 17011 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (717) 761-2633 Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of each exchange on which registered Common Stock, $1.00 par value New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ፤ No អ Indicate by check mark if the registrant is not required to file reports pursuant to section 13 or section 15(d) of the Exchange Act. Yes អ No ፤ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.