Treatment of Pulmonary Exacerbations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Allergic Bronchopulmonary Aspergillosis Revealing Asthma
CASE REPORT published: 22 June 2021 doi: 10.3389/fimmu.2021.695954 Case Report: Allergic Bronchopulmonary Aspergillosis Revealing Asthma Houda Snen 1,2*, Aicha Kallel 2,3*, Hana Blibech 1,2, Sana Jemel 2,3, Nozha Ben Salah 1,2, Sonia Marouen 3, Nadia Mehiri 1,2, Slah Belhaj 3, Bechir Louzir 1,2 and Kalthoum Kallel 2,3 1 Pulmonary Department, Hospital Mongi Slim, La Marsa, Tunisia, 2 Faculty of Medicine, Tunis El Manar University, Tunis, Tunisia, 3 Parasitology and Mycology Department, La Rabta Hospital, Tunis, Tunisia Allergic bronchopulmonary aspergillosis (ABPA) is an immunological pulmonary disorder caused by hypersensitivity to Aspergillus which colonizes the airways of patients with asthma and cystic fibrosis. Its diagnosis could be difficult in some cases due to atypical Edited by: presentations especially when there is no medical history of asthma. Treatment of ABPA is Brian Stephen Eley, frequently associated to side effects but cumulated drug toxicity due to different molecules University of Cape Town, South Africa is rarely reported. An accurate choice among the different available molecules and Reviewed by: effective on ABPA is crucial. We report a case of ABPA in a woman without a known Shivank Singh, Southern Medical University, China history of asthma. She presented an acute bronchitis with wheezing dyspnea leading to an Richard B. Moss, acute respiratory failure. She was hospitalized in the intensive care unit. The Stanford University, United States bronchoscopy revealed a complete obstruction of the left primary bronchus by a sticky *Correspondence: Houda Snen greenish material. The culture of this material isolated Aspergillus fumigatus and that of [email protected] bronchial aspiration fluid isolated Pseudomonas aeruginosa. -

Symptoms Related to Asthma and Chronic Bronchitis in Three Areas of Sweden
Eur Respir J, 1994, 7, 2146–2153 Copyright ERS Journals Ltd 1994 DOI: 10.1183/09031936.94.07122146 European Respiratory Journal Printed in UK - all rights reserved ISSN 0903 - 1936 Symptoms related to asthma and chronic bronchitis in three areas of Sweden E. Björnsson*, P. Plaschke**, E. Norrman+, C. Janson*, B. Lundbäck+, A. Rosenhall+, N. Lindholm**, L. Rosenhall+, E. Berglund++, G. Boman* Symptoms related to asthma and chronic bronchitis in three areas of Sweden. E. Björnsson, *Dept of Lung Medicine and Asthma P. Plaschke, E. Norrman, C. Janson, B. Lundbäck, A. Rosenhall, N. Lindholm, L. Research Center, Akademiska sjukhu- Rosenhall, E. Berglund, G. Boman. ERS Journals Ltd 1994. set, Uppsala University, Uppsala, Sweden. ABSTRACT: Does the prevalence of respiratory symptoms differ between regions? **Asthma and Allergy Research Center, Sahlgren's Hospital, University of Göteborg, As a part of the European Community Respiratory Health Survey, we present data Göteborg, Sweden. +Dept of Pulmonary from an international questionnaire on asthma symptoms occurring during a 12 Medicine and Allergology, Univer- month period, smoking and symptoms of chronic bronchitis. The questionnaire was sity Hospital of Northern Sweden, Umeå, mailed to 10,800 persons aged 20–44 yrs living in three regions of Sweden (Västerbotten, Sweden. ++Dept of Pulmonary Medicine, Uppsala and Göteborg) with different environmental characteristics. The total Sahlgrenska University Hospital, Göteborg, response rate was 86%. Sweden. Wheezing was reported by 20.5%, and the combination of wheezing without a Correspondence: E. Björnsson, Dept of cold and wheezing with breathlessness by 7.4%. The use of asthma medication was Lung Medicine, Akademiska sjukhuset, S- reported by 5.3%. -

Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1
ISSN: 2572-4193 Gelpi et al. J Otolaryngol Rhinol 2018, 4:036 DOI: 10.23937/2572-4193.1510036 Volume 4 | Issue 1 Journal of Open Access Otolaryngology and Rhinology CASE REPORT Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1 1 Check for University Hospitals Cleveland Medical Center, USA updates 2Case Western Reserve University School of Medicine, USA *Corresponding author: Mark Gelpi, MD, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA, Tel: (216)-844-8433, Fax: (216)-201-4479, E-mail: [email protected] paranasal sinuses [1,4]. Nasal symptoms in CF patients Abstract occur early, manifesting between 5-14 years of age, and Cystic fibrosis (CF) is a multisystem disease that can have represent a life-long problem in this population [5]. Pa- significant sinonasal manifestations. Viscous secretions are one of several factors in CF that result in chronic sinona- tients with CF can develop thick nasal secretions con- sal pathology, such as sinusitis, polyposis, congestion, and tributing to chronic rhinosinusitis (CRS), nasal conges- obstructive crusting. Persistent discomfort and nasal man- tion, nasal polyposis, headaches, and hyposmia [6-8]. ifestations of this disease significantly affect quality of life. Sinonasal symptoms of CF are managed medically with Digital manipulation and removal of crusting by the patient in an attempt to alleviate the discomfort can have unfore- topical agents and antibiotics, however surgery can be seen damaging consequences. We present one such case warranted due to the chronic and refractory nature of and investigate other cases of septal damage secondary to the symptoms, with 20-25% of CF patients undergoing digital trauma, as well as discuss the importance of sinona- sinus surgery in their lifetime [8]. -

Cryptogenic Organizing Pneumonia—Results of Treatment with Clarithromycin Versus Corticosteroids—Observational Study
RESEARCH ARTICLE Cryptogenic organizing pneumoniaÐResults of treatment with clarithromycin versus corticosteroidsÐObservational study Elżbieta Radzikowska1*, Elżbieta Wiatr1☯, Renata Langfort2³, Iwona Bestry3³, Agnieszka Skoczylas4, Ewa Szczepulska-Wo jcik2³, Dariusz Gawryluk1☯, Piotr Rudziński5³, Joanna Chorostowska-Wynimko6³, Kazimierz Roszkowski-Śliż1³ 1 III Department of Lung Disease National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland, 2 Pathology Department National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland, 3 Radiology Department National Tuberculosis and Lung Diseases Research Institute, Warsaw, a1111111111 Poland, 4 Geriatrics Department National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, a1111111111 Poland, 5 Thoracic Surgery Department National Tuberculosis and Lung Diseases Research Institute, a1111111111 Warsaw, Poland, 6 Laboratory of Molecular Diagnostics and Immunology National Tuberculosis and Lung Diseases Research Institute, Warsaw, Poland a1111111111 a1111111111 ☯ These authors contributed equally to this work. ³ These authors also contributed equally to this work. * [email protected] OPEN ACCESS Abstract Citation: Radzikowska E, Wiatr E, Langfort R, Bestry I, Skoczylas A, Szczepulska-WoÂjcik E, et al. (2017) Cryptogenic organizing pneumoniaÐ Background Results of treatment with clarithromycin versus Cryptogenic organizing pneumonia (COP) is a clinicopathological syndrome of unknown ori- corticosteroidsÐObservational study. PLoS ONE 12(9): e0184739. -

Acute (Serious) Bronchitis
Acute (serious) Bronchitis This is an infection of the air tubes that go down to your lungs. It often follows a cold or the flu. Most people do not need treatment for this. The infection normally goes away in 7-10 days. We make every effort to make sure the information is correct (right). However, we cannot be responsible for any actions as a result of using this information. Getting Acute Bronchitis How the lungs work Your lungs are like two large sponges filled with tubes. As you breathe in, you suck oxygen through your nose and mouth into a tube in your neck. Bacteria and viruses in the air can travel into your lungs. Normally, this does not cause a problem as your body kills the bacteria, or viruses. However, sometimes infection can get through. If you smoke or if you have had another illness, infections are more likely to get through. Acute Bronchitis Acute bronchitis is when the large airways (breathing tubes) to the lungs get inflamed (swollen and sore). The infection makes the airways swell and you get a build up of phlegm (thick mucus). Coughing is a way of getting the phlegm out of your airways. The cough can sometimes last for up to 3 weeks. Acute Bronchitis usually goes away on its own and does not need treatment. We make every effort to make sure the information is correct (right). However, we cannot be responsible for any actions as a result of using this information. Symptoms (feelings that show you may have the illness) Symptoms of Acute Bronchitis include: • A chesty cough • Coughing up mucus, which is usually yellow, or green • Breathlessness when doing more energetic activities • Wheeziness • Dry mouth • High temperature • Headache • Loss of appetite The cough usually lasts between 7-10 days. -

Does Cystic Fibrosis Constitute an Advantage in COVID-19 Infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli*
Bezzerri et al. Italian Journal of Pediatrics (2020) 46:143 https://doi.org/10.1186/s13052-020-00909-1 LETTER TO THE EDITOR Open Access Does cystic fibrosis constitute an advantage in COVID-19 infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli* Abstract The Veneto region is one of the most affected Italian regions by COVID-19. Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), may constitute a risk factor in COVID-19. Moreover, respiratory viruses were generally associated with severe pulmonary impairment in cystic fibrosis (CF). We would have therefore expected numerous cases of severe COVID-19 among the CF population. Surprisingly, we found that CF patients were significantly protected against infection by SARS-CoV-2. We discussed this aspect formulating some reasonable theories. Keywords: Cystic fibrosis, SARS-CoV-2, Covid-19, Azythromycin, DNase Introduction status, one would surmise that CF patients would be at The comorbidities of obesity, hypertension, diabetes, an increased risk of developing severe COVID-19 illness. heart failure, and chronic lung disease have been associ- ated with poor outcome in coronavirus disease 2019 Methods (COVID-19) [1]. Once Severe Acute Respiratory Syn- We conducted a retrospective study of 532 CF patients – drome (SARS) Coronavirus (CoV)-2 has infected host followed at the Cystic Fibrosis Center of Verona, Italy. cells, excessive inflammatory and thrombotic processes SARS-CoV-2 positivity was tested by collecting com- take place. A cytokine storm release with markedly ele- bined nose-throat swabs and subsequent Real-Time PCR vated IL-6 levels are associated with increased lethality using the Nimbus MuDT tm (Seegene, Seoul, South [2]. -

Allergic Bronchopulmonary Aspergillosis and Severe Asthma with Fungal Sensitisation
Allergic Bronchopulmonary Aspergillosis and Severe Asthma with Fungal Sensitisation Dr Rohit Bazaz National Aspergillosis Centre, UK Manchester University NHS Foundation Trust/University of Manchester ~ ABPA -a41'1 Severe asthma wl'th funga I Siens itisat i on Subacute IA Chronic pulmonary aspergillosjs Simp 1Ie a:spe rgmoma As r§i · bronchitis I ram une dysfu net Ion Lun· damage Immu11e hypce ractivitv Figure 1 In t@rarctfo n of Aspergillus Vliith host. ABP A, aHerg tc broncho pu~ mo na my as µe rgi ~fos lis; IA, i nvas we as ?@rgiH os 5. MANCHl·.'>I ER J:-\2 I Kosmidis, Denning . Thorax 2015;70:270–277. doi:10.1136/thoraxjnl-2014-206291 Allergic Fungal Airway Disease Phenotypes I[ Asthma AAFS SAFS ABPA-S AAFS-asthma associated with fu ngaIsensitization SAFS-severe asthma with funga l sensitization ABPA-S-seropositive a llergic bronchopulmonary aspergi ll osis AB PA-CB-all ergic bronchopulmonary aspergi ll osis with central bronchiectasis Agarwal R, CurrAlfergy Asthma Rep 2011;11:403 Woolnough K et a l, Curr Opin Pulm Med 2015;21:39 9 Stanford Lucile Packard ~ Children's. Health Children's. Hospital CJ Scanford l MEDICINE Stanford MANCHl·.'>I ER J:-\2 I Aspergi 11 us Sensitisation • Skin testing/specific lgE • Surface hydroph,obins - RodA • 30% of patients with asthma • 13% p.atients with COPD • 65% patients with CF MANCHl·.'>I ER J:-\2 I Alternar1a• ABPA •· .ABPA is an exagg·erated response ofthe imm1une system1 to AspergUlus • Com1pUcatio n of asthm1a and cystic f ibrosis (rarell·y TH2 driven COPD o r no identif ied p1 rior resp1 iratory d isease) • ABPA as a comp1 Ucation of asth ma affects around 2.5% of adullts. -

Obliterative Bronchiolitis, Cryptogenic Organising Pneumonitis and Bronchiolitis Obliterans Organizing Pneumonia: Three Names for Two Different Conditions
Eur Reaplr J EDITORIAL 1991, 4, 774-775 Obliterative bronchiolitis, cryptogenic organising pneumonitis and bronchiolitis obliterans organizing pneumonia: three names for two different conditions R.M. du Bois, O.M. Geddes Over the last five years, increasing confusion has has been applied to conditions in which airflow obstruc developed over the use of the terms "bronchiolitis tion is prominent and in which response to treatment is obliterans" and "bronchiolitis obliterans organizing poor. pneumonia". The confusion stems largely from the common use of the term "bronchiolitis obliterans" or "obliterative bronchiolitis" in the diagnostic labels applied "Cryptogenic organizing pneumonitis" or "bronchi· to two entities which are quite distinct clinically but which otitis obliterans organizing pneumonia" (BOOP) bear certain resemblances histologically. Cryptogenic organizing pneumonitis was first described by DAVISON et al. [7] in 1983. The clinical syndrome ObUterative bronchiolitis consisted of breathlessness, malaise, fever, high erythrocyte sedimentation rate (ESR), pneumonic In 1977, GEODES et al. [1] reported the case histories shadowing on chest radiograph with a restrictive of six patients whose clinical condition was characterized pulmonary function defect and low gas transfer coeffi by airways obliteration in association with rheumatoid cient. On histological examination of lung biopsy mate· arthritis. The striking clinical features were of rapidly rial, the typical and distinguishing feature was the progressive breathlessness and the fmding on examination presence of connective tissue within the alveoli, alveolar of a high-pitched mid-inspiratory squeak heard over the ducts and, occasionally, in respiratory bronchioles. This lung fields. Chest radiographs showed hyperinflated lungs connective tissue consisted of "loosely woven fibres of but were otherwise normal. -

Bronchodilator Responsiveness in Children with Cystic Fibrosis and Allergic Bronchopulmonary Aspergillosis
AGORA | RESEARCH LETTER Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis Mordechai Pollak 1, Michelle Shaw2, David Wilson1, Hartmut Grasemann1,2 and Felix Ratjen1,2 Affiliations: 1Division of Respiratory Medicine, Hospital for Sick Children, Toronto, ON, Canada. 2Translational Medicine, Sickkids Research Institute, Toronto, ON, Canada. Correspondence: Mordechai Pollak, Hospital for Sick Children, SickKids Learning Institute, Respiratory Medicine, 555 University Ave, Toronto, ON M5G 1X8, Canada. E-mail: [email protected] @ERSpublications CF patients with a new diagnosis of ABPA had a similar BD response, compared to CF patients with acute lung function deterioration from other causes. BD response testing did not help differentiating ABPA from other causes of lung function deterioration. https://bit.ly/39Oegnh Cite this article as: Pollak M, Shaw M, Wilson D, et al. Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis. Eur Respir J 2020; 56: 2000175 [https://doi.org/ 10.1183/13993003.00175-2020]. This single-page version can be shared freely online. To the Editor: Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease that occurs in approximately 9% of children with cystic fibrosis (CF) [1]. While ABPA is commonly associated with worsening lung function, differentiating ABPA from other causes of pulmonary function decline often poses a clinical challenge. This is reflected by major differences among the various diagnostic criteria for ABPA that have been suggested to date [2–5]. A positive bronchodilator response (BDR) is characteristic for asthma which is a common co-morbidity in CF patients, but whether this is helpful in differentiating ABPA from other causes of deterioration in lung function is currently unclear. -

Infection Control Recommendations for Patients with Cystic Fibrosis
S6 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY May 2003 INFECTION CONTROL RECOMMENDATIONS FOR PATIENTS WITH CYSTIC FIBROSIS: MICROBIOLOGY, IMPORTANT PATHOGENS, AND INFECTION CONTROL PRACTICES TO PREVENT PATIENT-TO-PATIENT TRANSMISSION Lisa Saiman, MD, MPH; Jane Siegel, MD; and the Cystic Fibrosis Foundation Consensus Conference on Infection Control Participants EXECUTIVE SUMMARY (d) The previously published HICPAC/CDC guidelines for Infection Control Recommendations for Patients With prevention of healthcare-associated infections have not Cystic Fibrosis: Microbiology, Important Pathogens, and included background information and recommenda- Infection Control Practices to Prevent Patient-to-Patient tions for the specific circumstances of patients with CF. Transmission updates, expands, and replaces the con- Thus, specific guidelines for CF patients are needed. sensus statement, Microbiology and Infectious Disease in (e) The link between acquisition of pathogens and morbidity Cystic Fibrosis published in 1994.1 This consensus docu- and mortality is well established. Prevention of acquisi- ment presents background data and evidence-based rec- tion of specific pathogens may further improve the mean ommendations for practices that are intended to decrease survival of CF patients, which has increased to 33.4 years the risk of transmission of respiratory pathogens among in 2001.3-9 CF patients from contaminated respiratory therapy equip- A multidisciplinary committee consisting of health- ment or the contaminated environment and thereby reduce care professionals from the United States, Canada, and the burden of respiratory illness. Included are recommen- Europe with experience in CF care and healthcare epi- dations applicable in the acute care hospital, ambulatory, demiology/infection control reviewed the relevant litera- home care, and selected non-healthcare settings. -
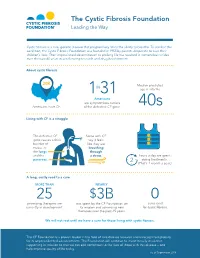
The Cystic Fibrosis Foundation Leading the Way
The Cystic Fibrosis Foundation Leading the Way Cystic fibrosis is a rare, genetic disease that progressively limits the ability to breathe. To combat this condition, the Cystic Fibrosis Foundation was founded in 1955 by parents desperate to save their children’s lives. Their impassioned determination to prolong life has resulted in tremendous strides over the past 60 years in accelerating research and drug development. About cystic fibrosis Median predicted age is into the Americans are symptomless carriers Americans have CF. of the defective CF gene. Living with CF is a struggle The defective CF Some with CF gene causes a thick say it feels buildup of like they are mucus in breathing the lungs through and the a straw. hours a day are spent pancreas. doing treatments. (That’s 1 month a year.) A long, costly road to a cure promising therapies are was spent by the CF Foundation on cures exist currently in development. its mission and advancing new for cystic fibrosis. therapies over the past 25 years. We will not rest until we have a cure for those living with cystic fibrosis. The CF Foundation is a proven leader in the field of rare disease research and is recognized globally for its unprecedented advancements. The Foundation will continue to invest heavily in science supporting its mission so that we can add tomorrows to the lives of those with this disease – and help improve quality of life today. As of September 2018 Jordan, age 22 While people with CF are living longer than in the past, we still lose precious young lives every day. -

SAR156597 in Idiopathic Pulmonary Fibrosis: a Phase 2, Placebo-Controlled Study (DRI11772)
ERJ Express. Published on October 18, 2018 as doi: 10.1183/13993003.01130-2018 Early View Original article SAR156597 in idiopathic pulmonary fibrosis: a phase 2, placebo-controlled study (DRI11772) Ganesh Raghu, Luca Richeldi, Bruno Crestani, Peter Wung, Raphael Bejuit, Corinne Esperet, Christian Antoni, Christina Soubrane Please cite this article as: Raghu G, Richeldi L, Crestani B, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2, placebo-controlled study (DRI11772). Eur Respir J 2018; in press (https://doi.org/10.1183/13993003.01130-2018). This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online. Copyright ©ERS 2018 Copyright 2018 by the European Respiratory Society. SAR156597 in idiopathic pulmonary fibrosis: a phase 2, placebo-controlled study (DRI11772) Ganesh Raghu1, Luca Richeldi2, Bruno Crestani3, Peter Wung4, Raphael Bejuit5, Corinne Esperet5, Christian Antoni5 and Christina Soubrane5 Affiliations: 1Center for Interstitial Lung Diseases, Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington Medical Center, Seattle, WA, USA. 2Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolicà del Sacro Cuore, Roma, Italy. 3APHP, Hôpital Bichat, Service de Pneumologie A, Centre de Référence Constitutif des Maladies Pulmonaires Rares, DHU FIRE, Paris, France; Université Paris Diderot, Sorbonne Paris Cité; INSERM Unité 1152, Paris, France. 4Medical Operations, Sanofi R&D, Bridgewater, NJ, USA. 5Immunology and Inflammation Therapeutic Area, Sanofi R&D, Paris, France.