Cryptogenic Organizing Pneumonia—Results of Treatment with Clarithromycin Versus Corticosteroids—Observational Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality
Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality Please note, this report is designed for double-sided printing American Lung Association Epidemiology and Statistics Unit Research and Health Education Division March 2013 Page intentionally left blank Table of Contents COPD Mortality, 1999-2009 COPD Prevalence, 1999-2011 COPD Hospital Discharges, 1999-2010 Glossary and References List of Tables Table 1: COPD – Number of Deaths by Ethnic Origin and Sex, 1999-2009 Figure 1: COPD – Number of Deaths by Sex, 1999-2009 Figure 2: COPD – Age-Adjusted Death Rates by Ethnic Origin and Sex, 2009 Table 2: COPD – Age-Adjusted Death Rate per 100,000 Population by Ethnic Origin and Sex, 1999- 2009 Figure 3: COPD – Deaths and Age-Adjusted Death Rate by Sex, 2009 Figure 4: COPD – Diagnosed Cases and Evidence of Impaired Lung Function Figure 5: Chronic Bronchitis – Prevalence Rates per 1,000, 2011 Table 3: Chronic Bronchitis – Number of Conditions and Prevalence Rate per 1,000 Population by Ethnic Origin, Sex and Age, 1999-2011 Figure 6: Emphysema – Prevalence Rates per 1,000, 2011 Table 4: Emphysema – Number of Conditions and Prevalence Rate per 1,000 Population by Ethnic Origin, Sex and Age, 1997-2011 Table 5: COPD – Adult Prevalence by Sex and State, 2011 Figure 8: COPD – Age-Adjusted Prevalence in Adults by State, 2011 Table 6: Characteristics Among Those Reporting a Diagnosis of COPD by State (%), 2011 Figure 9: COPD – First-Listed Hospital Discharge Rates per 10,000, 2010 Table 7: COPD – Number of First-Listed Hospital Discharges and Rate per 10,000 Population by Race, Sex and Age, 1999-2010 Figure 9: National Projected Annual Cost of COPD, 2010 Introduction Chronic obstructive pulmonary disease (COPD) is a term which refers to a large group of lung diseases characterized by obstruction of air flow that interferes with normal breathing. -
Chronic Obstructive Lung Disease &Bronchiectasis
PLEASE CHECK Editing file BEFORE! Chronic Obstructive lung Disease & Bronchiectasis ★ Objectives: 1. Definition of the two conditions 2. Clinical and radiological diagnosis 3. Differential diagnosis 4. General outline of management 5. Create a link to 341 clinical teaching ★ Resources Used in This lecture: Davidson, Guyton and Becker step 1 lecture notes Done by: Mohammad Alkharraz Contact us at: [email protected] Chronic Obstructive Pulmonary Diseases (COPD) COPD contains two diseases which are chronic bronchitis and emphysema ■ COPD is classified under obstructive pulmonary diseases along with other (no kidding!) diseases such as asthma and bronchiectasis ■ Chronic bronchitis and emphysema are grouped together under COPD because they are both mainly caused by smoking and they usually present together. ■ Brainless pathology textbooks (Robbins) try to confuse us students with “Pink Puffers” and “Blue Bloaters”. Forget about that as it is not clinically relevant1. Let us quickly point out some points (pathology) that are relevant to each disease (chronic bronchitis and emphysema) and then we will discuss the clinical presentation, management, etc. of COPD Chronic bronchitis It is mainly a clinical diagnosis patients cough up lots of sputum for a long period of time “3 months per year for at least 2 consecutive years2” ● Pathogenesis: Cigarette smoke causes hyperplasia of mucus glands which increase the secretion of mucus → mucus plugs cause obstruction of bronchioles→ COPD Emphysema It is mainly a pathological diagnosis. ● Pathogenesis: 1. Pollutants (smoking)→ increased inflammatory mediators in the lung that destroy the lung parenchyma (trypsin and elastase) 2. We have defense mechanisms to fight these inflammatory mediators (alpha1 antitrypsin) ○ However, the amount of inflammatory mediators exceeds our ability to counteract them 3. -

Allergic Bronchopulmonary Aspergillosis As a Cause of Bronchial Asthma in Children
Egypt J Pediatr Allergy Immunol 2012;10(2):95-100. Original article Allergic bronchopulmonary aspergillosis as a cause of bronchial asthma in children Background: Allergic bronchopulmonary aspergillosis (ABPA) occurs in Dina Shokry, patients with asthma and cystic fibrosis. When aspergillus fumigatus spores Ashgan A. are inhaled they grow in bronchial mucous as hyphae. It occurs in non Alghobashy, immunocompromised patients and belongs to the hypersensitivity disorders Heba H. Gawish*, induced by Aspergillus. Objective: To diagnose cases of allergic bronchopulmonary aspergillosis among asthmatic children and define the Manal M. El-Gerby* association between the clinical and laboratory findings of aspergillus fumigatus (AF) and bronchial asthma. Methods: Eighty asthmatic children were recruited in this study and divided into 50 atopic and 30 non-atopic Departments of children. The following were done: skin prick test for aspergillus fumigatus Pediatrics and and other allergens, measurement of serum total IgE, specific serum Clinical Pathology*, aspergillus fumigatus antibody titer IgG and IgE (AF specific IgG and IgE) Faculty of Medicine, and absolute eosinophilic count. Results: ABPA occurred only in atopic Zagazig University, asthmatics, it was more prevalent with decreased forced expiratory volume Egypt. at the first second (FEV1). Prolonged duration of asthma and steroid dependency were associated with ABPA. AF specific IgE and IgG were higher in the atopic group, they were higher in Aspergillus fumigatus skin Correspondence: prick test positive children than negative ones .Wheal diameter of skin prick Dina Shokry, test had a significant relation to the level of AF IgE titer. Skin prick test Department of positive cases for aspergillus fumigatus was observed in 32% of atopic Pediatrics, Faculty of asthmatic children. -

Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1
ISSN: 2572-4193 Gelpi et al. J Otolaryngol Rhinol 2018, 4:036 DOI: 10.23937/2572-4193.1510036 Volume 4 | Issue 1 Journal of Open Access Otolaryngology and Rhinology CASE REPORT Rhinotillexomania in a Cystic Fibrosis Patient Resulting in Septal Perforation Mark Gelpi1*, Emily N Ahadizadeh1,2, Brian D’Anzaa1 and Kenneth Rodriguez1 1 Check for University Hospitals Cleveland Medical Center, USA updates 2Case Western Reserve University School of Medicine, USA *Corresponding author: Mark Gelpi, MD, University Hospitals Cleveland Medical Center, 11100 Euclid Avenue, Cleveland, OH 44106, USA, Tel: (216)-844-8433, Fax: (216)-201-4479, E-mail: [email protected] paranasal sinuses [1,4]. Nasal symptoms in CF patients Abstract occur early, manifesting between 5-14 years of age, and Cystic fibrosis (CF) is a multisystem disease that can have represent a life-long problem in this population [5]. Pa- significant sinonasal manifestations. Viscous secretions are one of several factors in CF that result in chronic sinona- tients with CF can develop thick nasal secretions con- sal pathology, such as sinusitis, polyposis, congestion, and tributing to chronic rhinosinusitis (CRS), nasal conges- obstructive crusting. Persistent discomfort and nasal man- tion, nasal polyposis, headaches, and hyposmia [6-8]. ifestations of this disease significantly affect quality of life. Sinonasal symptoms of CF are managed medically with Digital manipulation and removal of crusting by the patient in an attempt to alleviate the discomfort can have unfore- topical agents and antibiotics, however surgery can be seen damaging consequences. We present one such case warranted due to the chronic and refractory nature of and investigate other cases of septal damage secondary to the symptoms, with 20-25% of CF patients undergoing digital trauma, as well as discuss the importance of sinona- sinus surgery in their lifetime [8]. -

Chronic Obstructive Pulmonary Disease (Copd) in the Americas
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) IN THE AMERICAS KEY STATISTICS FOR THE AMERICAS An estimated 13.2 million people live with COPD (1). COPD caused over 235,000 deaths in 2010, ranking as the sixth leading cause of death (2). 7 in 10 COPD deaths are attributable to tobacco (3). In 2012, COPD was responsible for the loss of 8.3 million disability-adjusted life years (DALYs) (3). KEY MESSAGES CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) IS A LEADING CAUSE OF MORBIDITY AND MORTALITY IN THE 1 AMERICAS, REPRESENTING AN IMPORTANT PUBLIC HEALTH CHALLENGE THAT IS BOTH PREVENTABLE AND TREATABLE. COPD is an incurable disease, characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and the lungs. Common symptoms include breathlessness, abnormal sputum and a chronic cough. Exacerbations and comorbidities – such as cardiovascular diseases, skeletal muscle dysfunction, metabolic syndrome, osteoporosis, depression and lung cancer – contribute to the overall severity of individual patients (4,5). In 2010, COPD accounted for over 235,000 deaths in the Americas, ranking as the sixth leading cause of mortality regionally. About 23% of these deaths occurred prematurely, in people aged 30-69 years (2). An estimated 13.2 million people live with COPD in the region (1). Many people suffer from this disease for years, experiencing disa- bility and major adverse effects on their quality of life (5,6). In 2012, COPD was responsible for the loss of 8.3 million DALYs, representing the seventh leading cause of disability-adjusted life years lost (DALYs) in the Americas, with one DALY representing the loss of the equivalent of one year of full health (3,6). -

Does Cystic Fibrosis Constitute an Advantage in COVID-19 Infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli*
Bezzerri et al. Italian Journal of Pediatrics (2020) 46:143 https://doi.org/10.1186/s13052-020-00909-1 LETTER TO THE EDITOR Open Access Does cystic fibrosis constitute an advantage in COVID-19 infection? Valentino Bezzerri, Francesca Lucca, Sonia Volpi and Marco Cipolli* Abstract The Veneto region is one of the most affected Italian regions by COVID-19. Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), may constitute a risk factor in COVID-19. Moreover, respiratory viruses were generally associated with severe pulmonary impairment in cystic fibrosis (CF). We would have therefore expected numerous cases of severe COVID-19 among the CF population. Surprisingly, we found that CF patients were significantly protected against infection by SARS-CoV-2. We discussed this aspect formulating some reasonable theories. Keywords: Cystic fibrosis, SARS-CoV-2, Covid-19, Azythromycin, DNase Introduction status, one would surmise that CF patients would be at The comorbidities of obesity, hypertension, diabetes, an increased risk of developing severe COVID-19 illness. heart failure, and chronic lung disease have been associ- ated with poor outcome in coronavirus disease 2019 Methods (COVID-19) [1]. Once Severe Acute Respiratory Syn- We conducted a retrospective study of 532 CF patients – drome (SARS) Coronavirus (CoV)-2 has infected host followed at the Cystic Fibrosis Center of Verona, Italy. cells, excessive inflammatory and thrombotic processes SARS-CoV-2 positivity was tested by collecting com- take place. A cytokine storm release with markedly ele- bined nose-throat swabs and subsequent Real-Time PCR vated IL-6 levels are associated with increased lethality using the Nimbus MuDT tm (Seegene, Seoul, South [2]. -

Interstitial Lung Disease—Raising the Index of Suspicion in Primary Care
www.nature.com/npjpcrm All rights reserved 2055-1010/14 PERSPECTIVE OPEN Interstitial lung disease: raising the index of suspicion in primary care Joseph D Zibrak1 and David Price2 Interstitial lung disease (ILD) describes a group of diseases that cause progressive scarring of the lung tissue through inflammation and fibrosis. The most common form of ILD is idiopathic pulmonary fibrosis, which has a poor prognosis. ILD is rare and mainly a disease of the middle-aged and elderly. The symptoms of ILD—chronic dyspnoea and cough—are easily confused with the symptoms of more common diseases, particularly chronic obstructive pulmonary disease and heart failure. ILD is infrequently seen in primary care and a precise diagnosis of these disorders can be challenging for physicians who rarely encounter them. Confirming a diagnosis of ILD requires specialist expertise and review of a high-resolution computed tomography scan (HRCT). Primary care physicians (PCPs) play a key role in facilitating the diagnosis of ILD by referring patients with concerning symptoms to a pulmonologist and, in some cases, by ordering HRCTs. In our article, we highlight the importance of prompt diagnosis of ILD and describe the circumstances in which a PCP’s suspicion for ILD should be raised in a patient presenting with chronic dyspnoea on exertion, once more common causes of dyspnoea have been investigated and excluded. npj Primary Care Respiratory Medicine (2014) 24, 14054; doi:10.1038/npjpcrm.2014.54; published online 11 September 2014 INTRODUCTION emphysema, in which the airways of the lungs become narrowed Interstitial lung disease (ILD) is an umbrella term, synonymous or blocked so the patient cannot exhale completely. -

Bronchodilator Responsiveness in Children with Cystic Fibrosis and Allergic Bronchopulmonary Aspergillosis
AGORA | RESEARCH LETTER Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis Mordechai Pollak 1, Michelle Shaw2, David Wilson1, Hartmut Grasemann1,2 and Felix Ratjen1,2 Affiliations: 1Division of Respiratory Medicine, Hospital for Sick Children, Toronto, ON, Canada. 2Translational Medicine, Sickkids Research Institute, Toronto, ON, Canada. Correspondence: Mordechai Pollak, Hospital for Sick Children, SickKids Learning Institute, Respiratory Medicine, 555 University Ave, Toronto, ON M5G 1X8, Canada. E-mail: [email protected] @ERSpublications CF patients with a new diagnosis of ABPA had a similar BD response, compared to CF patients with acute lung function deterioration from other causes. BD response testing did not help differentiating ABPA from other causes of lung function deterioration. https://bit.ly/39Oegnh Cite this article as: Pollak M, Shaw M, Wilson D, et al. Bronchodilator responsiveness in children with cystic fibrosis and allergic bronchopulmonary aspergillosis. Eur Respir J 2020; 56: 2000175 [https://doi.org/ 10.1183/13993003.00175-2020]. This single-page version can be shared freely online. To the Editor: Allergic bronchopulmonary aspergillosis (ABPA) is a hypersensitivity lung disease that occurs in approximately 9% of children with cystic fibrosis (CF) [1]. While ABPA is commonly associated with worsening lung function, differentiating ABPA from other causes of pulmonary function decline often poses a clinical challenge. This is reflected by major differences among the various diagnostic criteria for ABPA that have been suggested to date [2–5]. A positive bronchodilator response (BDR) is characteristic for asthma which is a common co-morbidity in CF patients, but whether this is helpful in differentiating ABPA from other causes of deterioration in lung function is currently unclear. -

Infection Control Recommendations for Patients with Cystic Fibrosis
S6 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY May 2003 INFECTION CONTROL RECOMMENDATIONS FOR PATIENTS WITH CYSTIC FIBROSIS: MICROBIOLOGY, IMPORTANT PATHOGENS, AND INFECTION CONTROL PRACTICES TO PREVENT PATIENT-TO-PATIENT TRANSMISSION Lisa Saiman, MD, MPH; Jane Siegel, MD; and the Cystic Fibrosis Foundation Consensus Conference on Infection Control Participants EXECUTIVE SUMMARY (d) The previously published HICPAC/CDC guidelines for Infection Control Recommendations for Patients With prevention of healthcare-associated infections have not Cystic Fibrosis: Microbiology, Important Pathogens, and included background information and recommenda- Infection Control Practices to Prevent Patient-to-Patient tions for the specific circumstances of patients with CF. Transmission updates, expands, and replaces the con- Thus, specific guidelines for CF patients are needed. sensus statement, Microbiology and Infectious Disease in (e) The link between acquisition of pathogens and morbidity Cystic Fibrosis published in 1994.1 This consensus docu- and mortality is well established. Prevention of acquisi- ment presents background data and evidence-based rec- tion of specific pathogens may further improve the mean ommendations for practices that are intended to decrease survival of CF patients, which has increased to 33.4 years the risk of transmission of respiratory pathogens among in 2001.3-9 CF patients from contaminated respiratory therapy equip- A multidisciplinary committee consisting of health- ment or the contaminated environment and thereby reduce care professionals from the United States, Canada, and the burden of respiratory illness. Included are recommen- Europe with experience in CF care and healthcare epi- dations applicable in the acute care hospital, ambulatory, demiology/infection control reviewed the relevant litera- home care, and selected non-healthcare settings. -

Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders
88 XAOIOOIOO Radioaerosol Lung Scanning in Chronic Obstructive Pulmonary Disease (COPD) and Related Disorders Yong Whee Bahk, M.D. and Soo Kyo Chung, M.D. Introduction As a coordinated research project of the International Atomic Energy Agency (IAEA) a multicentre joint study on radioaerosol lung scan using the BARC nebulizer [1] has prospectively been carried out during 1988-1992 with the participation of 10 member countries in Asia [Bangladesh, China, India, Indonesia, Japan, Korea, Pakistan, Philippines, Singapore and Thailand]. The study was designed so that it would primarily cover chronic obstructive pulmonary disease (COPD) and the other related and common pulmonary diseases. The study also included normal controls and asymptomatic smokers. The purposes of this presentation are three fold: firstly, to document the useful- ness of the nebulizer and the validity of user's protocol in imaging COPD and other lung diseases; secondly, to discuss scan features of the individual COPD and other disorders studied and thirdly, to correlate scan alterations with radiographie find- ings. Before proceeding with a systematic analysis of aerosol scan patterns in the disease groups, we documented normal pattern. The next step was the assessment of scan features in those who had been smoking for more than several years but had no symptoms or signs referable to airways. The lung diseases we analyzed included COPD [emphysema, chronic bronchitis, asthma and bronchiectasis], bron- chial obstruction, compensatory overinflation and other common lung diseases such as lobar pneumonia, tuberculosis, interstitial fibrosis, diffuse panbronchiolitis, lung edema and primary and metastatic lung cancers. Lung embolism, inhalation burns and glue-sniffer's lung are seperately discussed by Dr. -
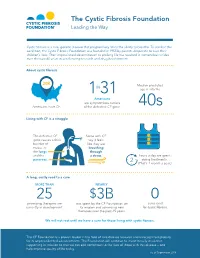
The Cystic Fibrosis Foundation Leading the Way
The Cystic Fibrosis Foundation Leading the Way Cystic fibrosis is a rare, genetic disease that progressively limits the ability to breathe. To combat this condition, the Cystic Fibrosis Foundation was founded in 1955 by parents desperate to save their children’s lives. Their impassioned determination to prolong life has resulted in tremendous strides over the past 60 years in accelerating research and drug development. About cystic fibrosis Median predicted age is into the Americans are symptomless carriers Americans have CF. of the defective CF gene. Living with CF is a struggle The defective CF Some with CF gene causes a thick say it feels buildup of like they are mucus in breathing the lungs through and the a straw. hours a day are spent pancreas. doing treatments. (That’s 1 month a year.) A long, costly road to a cure promising therapies are was spent by the CF Foundation on cures exist currently in development. its mission and advancing new for cystic fibrosis. therapies over the past 25 years. We will not rest until we have a cure for those living with cystic fibrosis. The CF Foundation is a proven leader in the field of rare disease research and is recognized globally for its unprecedented advancements. The Foundation will continue to invest heavily in science supporting its mission so that we can add tomorrows to the lives of those with this disease – and help improve quality of life today. As of September 2018 Jordan, age 22 While people with CF are living longer than in the past, we still lose precious young lives every day. -

Article First Wave of COVID-19 in French Patients with Cystic Fibrosis
Supplementary material First Wave of COVID-19 in French Patients with Cystic Fibrosis Harriet Corvol 1,2,*, Sandra de Miranda 3, Lydie Lemonnier 4, Astrid Kemgang 2, Martine Reynaud Gaubert 5,6, Raphael Chiron 7, Marie-Laure Dalphin 8, Isabelle Durieu 9, Jean-Christophe Dubus 10, Véronique Houdouin 11, Anne Prevotat 12, Sophie Ramel 13, Marine Revillion 14, Laurence Weiss 15, Loic Guillot 2, Pierre-Yves Boelle 16 and Pierre-Régis Burgel 17,18 on behalf the French Cystic Fibrosis Reference Network study group Supplementay material Expected Number and Age Distribution of COVID-19 in French Patients with Cystic Fibrosis We obtained the cumulated number of hospitalized COVID-19 cases in the French general population up to 30 June 2020. These data are open source, available from data.gouv.fr (https://www.data.gouv.fr/fr/datasets/r/41b9bd2a-b5b6-4271-8878-e45a8902ef00). Then, we obtained the probability of hospitalization upon SARS-Cov-2 infection by age from Table S1 in Salje et al. [21]. The numbers of people in each age class in France were obtained from census data (https://www.insee.fr/fr/statistiques/1892086?sommaire=1912926). From these data, we computed the extrapolated percentage of infections by age class up to 30 June 2020 in the general French population using proportionality rules. Finally, we computed the expected number of infections that would have been expected in the French CF population had the risk of infection been the same as in the general population. We used the number of CF patients from the national registry as the denominator to compute the expected number of SARS-Cov-2 CF cases [1].