Ecophysiological Interpretation of Oxygen Consumption Rates and Enzymatic Activities of Deep-Sea Copepods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bioluminescence of the Poecilostomatoid Copepod Oncaea Conifera
l MARINE ECOLOGY PROGRESS SERIES Published April 22 Mar. Ecol. Prog. Ser. Bioluminescence of the poecilostomatoid copepod Oncaea conifera Peter J. Herring1, M. I. ~atz~,N. J. ~annister~,E. A. widder4 ' Institute of Oceanographic Sciences, Deacon Laboratory, Brook Road Wormley, Surrey GU8 5UB, United Kingdom 'Marine Biology Research Division 0202, Scripps Institution of Oceanography, La Jolla, California 92093, USA School of Biological Sciences, University of Birmingham, Edgbaston. Birmingham B15 2TT, United Kingdom Harbor Branch Oceanographic Institution, 5600 Old Dixie Highway, Fort Pierce, Florida 34946, USA ABSTRACT: The small poecilostomatoid copepod Oncaea conifera Giesbrecht bears a large number of epidermal luminous glands, distributed primarily over the dorsal cephalosome and urosome. Bio- luminescence is produced in the form of short (80 to 200 ms duration) flashes from withrn each gland and there IS no visible secretory component. Nevertheless each gland opens to the exterior by a simple valved pore. Intact copepods can produce several hundred flashes before the luminescent system is exhausted. Individual flashes had a maximum measured flux of 7.5 X 10" quanta s ', and the flash rate follows the stimulus frequency up to 30 S" Video observations show that ind~vidualglands flash repeatedly and the flash propagates along their length. The gland gross morphology is highly variable although each gland appears to be unicellular. The cytoplasm contains an extensive endoplasmic reticulum. 0. conifera swims at Reynolds numbers of 10 to 50, and is normally associated with surfaces (e.g. marine snow). We suggest that the unique anatomical and physiological characteristics of the luminescent system arc related to the specialised ecological niche occupied by this species. -

The Effects of Density Gradients on the Distribution and Behavior of Copepods" (2018)
University of San Diego Digital USD Theses Theses and Dissertations Summer 8-31-2018 The ffecE ts of Density Gradients on the Distribution and Behavior of Copepods Connor Cayson University of San Diego Follow this and additional works at: https://digital.sandiego.edu/theses Part of the Marine Biology Commons, and the Oceanography Commons Digital USD Citation Cayson, Connor, "The Effects of Density Gradients on the Distribution and Behavior of Copepods" (2018). Theses. 33. https://digital.sandiego.edu/theses/33 This Thesis is brought to you for free and open access by the Theses and Dissertations at Digital USD. It has been accepted for inclusion in Theses by an authorized administrator of Digital USD. For more information, please contact [email protected]. UNIVERSITY OF SAN DIEGO San Diego The Effects of Density Gradients on the Distribution and Behavior of Copepods A thesis submitted in partial satisfaction of the requirements for the degree of Master of Science in Marine Science By Connor Payne Cayson Thesis Committee Jennifer Prairie, Ph.D., Chair Nathalie Reyns, Ph.D. Moira Décima, Ph.D. 2018 i The thesis of Connor Payne Cayson is approved by: _______________________________________________ Jennifer C. Prairie, Ph.D., Thesis Committee Chair University of San Diego _______________________________________________ Nathalie Reyns, Ph.D., Thesis Committee Member University of San Diego _______________________________________________ Moira Décima, Ph.D., Thesis Committee Member National Institute of Water and Atmospheric Research, Wellington, New Zealand University of San Diego San Diego 2018 ii Copyright © 2018 Connor Payne Cayson All Rights Reserved iii ACKNOWLEDGEMENTS First, I must thank my advisor, teacher, and committee chair, Dr. -

Ocean Deoxygenation and Copepods: Coping with Oxygen Minimum Zone Variability
Biogeosciences, 17, 2315–2339, 2020 https://doi.org/10.5194/bg-17-2315-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Ocean deoxygenation and copepods: coping with oxygen minimum zone variability Karen F. Wishner1, Brad Seibel2, and Dawn Outram1 1Graduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA 2College of Marine Science, University of South Florida, St. Petersburg, FL 33701, USA Correspondence: Karen F. Wishner ([email protected]) Received: 27 September 2019 – Discussion started: 28 October 2019 Revised: 31 March 2020 – Accepted: 2 April 2020 – Published: 24 April 2020 Abstract. Increasing deoxygenation (loss of oxygen) of compression concept). These distribution depths changed by the ocean, including expansion of oxygen minimum zones tens to hundreds of meters depending on the species, oxygen (OMZs), is a potentially important consequence of global profile, and phenomenon. For example, at the lower oxycline, warming. We examined present-day variability of vertical the depth of maximum abundance for Lucicutia hulsemannae distributions of 23 calanoid copepod species in the East- shifted from ∼ 600 to ∼ 800 m, and the depth of diapause for ern Tropical North Pacific (ETNP) living in locations with Eucalanus inermis shifted from ∼ 500 to ∼ 775 m, in an ex- different water column oxygen profiles and OMZ inten- panded OMZ compared to a thinner OMZ, but remained at sity (lowest oxygen concentration and its vertical extent in similar low oxygen levels in -

Tesis Estructura Comunitaria De Copepodos .Pdf
Universidad de Concepción Dirección de Postgrado Facultad de Ciencias Naturales y Oceanográficas Programa de Magister en Ciencias mención Oceanografía Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico Sur Oriental Tesis para optar al grado de Magíster en Ciencias con mención en Oceanografía PAMELA ANDREA FIERRO GONZÁLEZ CONCEPCIÓN-CHILE 2019 Profesora Guía: Pamela Hidalgo Díaz Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción Profesor Co-guía: Rubén Escribano Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción La Tesis de “Magister en Ciencias con mención en Oceanografía” titulada “Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico sur oriental”, de la Srta. “PAMELA ANDREA FIERRO GONZÁLEZ” y realizada bajo la Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, ha sido aprobada por la siguiente Comisión de Evaluación: Dra. Pamela Hidalgo Díaz Profesora Guía Universidad de Concepción Dr. Rubén Escribano Profesor Co-Guía Universidad de Concepción Dr. Samuel Hormazábal Miembro de la Comisión Evaluadora Pontificia Universidad Católica de Valparaíso Dr. Fabián Tapia Director Programa de Magister en Oceanografía Universidad de Concepción ii A Juan Carlos y Sebastián iii AGRADECIMIENTOS Agradezco a quienes con su colaboración y apoyo hicieron posible el desarrollo y término de esta tesis. En primer lugar, agradezco a los miembros de mi comisión de tesis. A mi profesora guía, Dra. Pamela Hidalgo, por apoyarme y guiarme en este largo camino de formación académica, por su gran calidad humana, contención y apoyo personal. -

Download The
A STUDY OF THE ECOLOGICAL RELATIONSHIPS AND TAXONOMIC STATUS OF TWO SPECIES OF THE GENUS CALANUS (CRUSTACEA: COPEPODA) by CHARLES D. WOODHOUSE, JR. B.A., University of California in Santa Barbara, 1962 M.A., University of Oregon, 1964 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in the Department of ZOOLOGY accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA April, 1971 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department The University of British Columbia Vancouver 8, Canada Date /^"^tP/y/. This thesis presents the results of an investigation on the relationships between populations of closely related animals un• der apparent sympatric conditions. The mechanisms found have particular application toward understanding the species problem among members of the free-swimming marine copepod genus Calanus that possess a toothed inner surface on the coxopodites of the fifth pair of swirnming legs. The investigation describes the morphology, distribution, and general ecology of two forms of toothed Calanus from the far eastern North Pacific Ocean. Morphological differences were es• tablished and used to distinguish both forms on the oasis of length, shape of the anterior surface of the cephalothorax, proportionate differences in segments of the urosome and fifth swimming legs, and by the degree of asymmetry in the fifth pair of swimming legs of males. -

Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans
Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans by Robert George Young A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Integrative Biology Guelph, Ontario, Canada © Robert George Young, March, 2016 ABSTRACT MOLECULAR SPECIES DELIMITATION AND BIOGEOGRAPHY OF CANADIAN MARINE PLANKTONIC CRUSTACEANS Robert George Young Advisors: University of Guelph, 2016 Dr. Sarah Adamowicz Dr. Cathryn Abbott Zooplankton are a major component of the marine environment in both diversity and biomass and are a crucial source of nutrients for organisms at higher trophic levels. Unfortunately, marine zooplankton biodiversity is not well known because of difficult morphological identifications and lack of taxonomic experts for many groups. In addition, the large taxonomic diversity present in plankton and low sampling coverage pose challenges in obtaining a better understanding of true zooplankton diversity. Molecular identification tools, like DNA barcoding, have been successfully used to identify marine planktonic specimens to a species. However, the behaviour of methods for specimen identification and species delimitation remain untested for taxonomically diverse and widely-distributed marine zooplanktonic groups. Using Canadian marine planktonic crustacean collections, I generated a multi-gene data set including COI-5P and 18S-V4 molecular markers of morphologically-identified Copepoda and Thecostraca (Multicrustacea: Hexanauplia) species. I used this data set to assess generalities in the genetic divergence patterns and to determine if a barcode gap exists separating interspecific and intraspecific molecular divergences, which can reliably delimit specimens into species. I then used this information to evaluate the North Pacific, Arctic, and North Atlantic biogeography of marine Calanoida (Hexanauplia: Copepoda) plankton. -

Observing Copepods Through a Genomic Lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace a Wyngaard6
Bron et al. Frontiers in Zoology 2011, 8:22 http://www.frontiersinzoology.com/content/8/1/22 DEBATE Open Access Observing copepods through a genomic lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace A Wyngaard6 Abstract Background: Copepods outnumber every other multicellular animal group. They are critical components of the world’s freshwater and marine ecosystems, sensitive indicators of local and global climate change, key ecosystem service providers, parasites and predators of economically important aquatic animals and potential vectors of waterborne disease. Copepods sustain the world fisheries that nourish and support human populations. Although genomic tools have transformed many areas of biological and biomedical research, their power to elucidate aspects of the biology, behavior and ecology of copepods has only recently begun to be exploited. Discussion: The extraordinary biological and ecological diversity of the subclass Copepoda provides both unique advantages for addressing key problems in aquatic systems and formidable challenges for developing a focused genomics strategy. This article provides an overview of genomic studies of copepods and discusses strategies for using genomics tools to address key questions at levels extending from individuals to ecosystems. Genomics can, for instance, help to decipher patterns of genome evolution such as those that occur during transitions from free living to symbiotic and parasitic lifestyles and can assist in the identification of genetic mechanisms and accompanying physiological changes associated with adaptation to new or physiologically challenging environments. The adaptive significance of the diversity in genome size and unique mechanisms of genome reorganization during development could similarly be explored. -

Ecdysteroids in the Oceanic Copepod Calanus Pacificus: Variation During Molt Cycle and Change Associated with Diapause
M 4648 MARINE ECOLOGY PROGRESS SERIES Vol. 257: 159–165, 2003 Published August 7 Mar Ecol Prog Ser 26 May 2003 CE: rs TS: jw PP: AL Ecdysteroids in the oceanic copepod Calanus pacificus: variation during molt cycle and change associated with diapause Catherine Lynn Johnson1, 2,* 1Integrative Oceanography Division, Scripps Institution of Oceanography, La Jolla, California 92093-0218, USA 2Present address: Department of Engineering Mathematics, Dalhousie University, PO Box 1000, Halifax, Nova Scotia B3J 2X4, Canada ABSTRACT: Development through the molt cycle in arthropods is characterized both by changes in exoskeleton morphology and by cyclical changes in ecdysteroids, i.e. molting hormones. This study describes for Calanus pacificus ecdysteroid variation during the molt cycle in the fifth copepodid (CV) stage and compares ecdysteroid content of diapausing and active field-collected CVs. In C. pacificus CVs, the pattern of ecdysteroid variation relative to the molt cycle phase is similar to that found in decapod crustacean larvae. Ecdysteroid levels are lowest in the postmolt, the first phase after molting, and then increase to a peak during early premolt. Ecdysteroid levels measured in field- collected diapausing C. pacificus CVs were lower than those in active CVs, as expected based on the predominance of the postmolt phase among diapausing CVs. Ecdysteroid analysis provides a faster, less labor-intensive alternative to jaw-phase analysis for identifying the molt phase of field-collected copepodids, and can provide valuable insights into their life-history status. Measurement of copepod hormonal status in situ may provide a direct means of determining the proximate cues for diapause induction and termination in oceanic copepods. -
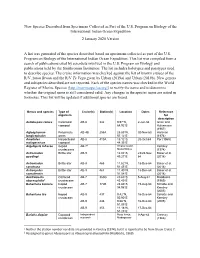
New Species Described from Specimens Collected As Part of the U.S
New Species Described from Specimens Collected as Part of the U.S. Program on Biology of the International Indian Ocean Expedition 2 January 2020 Version A list was generated of the species described based on specimens collected as part of the U.S. Program on Biology of the International Indian Ocean Expedition. This list was compiled from a search of publications cited by scientists involved in the U.S. Program on Biology and publications held by the Smithsonian Institution. The list includes holotypes and paratypes used to describe species. The cruise information was checked against the list of known cruises of the R/V Anton Bruun and the R/V Te Vega given by Urban (2019a) and Urban (2019b). New genera and subspecies described are not reported. Each of the species names was checked in the World Register of Marine Species (http://marinespecies.org/) to verify the name and to determine whether the original name is still considered valid. Any changes in the species name are noted in footnotes. This list will be updated if additional species are found. Genus and species Type of Cruise(s) Station(s) Location Dates Reference organism for description Aetideopsis retusa Calanaoid AB-6 342 9.97°S, 2-Jun-64 Grice and copepod 64.92°E Hulsemann (1967) Aglaophamus Polychaete AB-4B 255A 25.83°N, 30-Nov-63 Hartman longicephalus worm 57.12°E (1974) Ameliotes Harpacticoid AB-8 410A 15.12°S, 20-Oct-64 Por (1969) malagassicus copepod 44.35°E Argeiopsis inhacae isopod AB-71 Inhaca Island, Kensley crustaceans Mozambique (1974) Astrocladus Brittle star AB-9 12.83°S, 23/26-Nov- Baker et al. -

Four New Species of Heteromysis (Crustacea: Mysida) from Public Aquaria in Hawaii, Florida, and Western to Central Europe
European Journal of Taxonomy 735: 133–175 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.735.1247 www.europeanjournaloftaxonomy.eu 2021 · Wittmann K.J. & Abed-Navandi D. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:F1CE3697-319D-4D02-A99F-11A0E16A8743 Four new species of Heteromysis (Crustacea: Mysida) from public aquaria in Hawaii, Florida, and Western to Central Europe Karl J. WITTMANN 1,* & Daniel ABED-NAVANDI 2 1 Medical University of Vienna, Department of Environmental Health, Kinderspitalgasse 15, 1090 Vienna, Austria. 2 Haus des Meeres – Aqua Terra Zoo, Fritz Grünbaum Platz 1, 1060 Vienna, Austria. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:C90E7BC4-A27A-4B41-93F3-6224D17795FF 2 urn:lsid:zoobank.org:author:179B83B0-8C8B-4DF5-8986-4227B8E1BB9B Abstract. Four new species of the subgenus Heteromysis (Olivemysis) were detected in material from (sub)-tropical aquaria in six public aquarium institutions around the globe. Modifications of pleopods by spines represent the strongest structural complex used for differentiation within this subgenus: male pleopods 1–4 modified in H. smithsoniana sp. nov., male pleopods 2–4 plus female pleopod 2 in H. hornimani sp. nov. and H. waikikensis sp. nov. Additional important diagnostic characters are provided by the antennulae, uropods, and telson. The male of H. sixi sp. nov. represents a very rare case within the genus Heteromysis by having only pleopod 2 modified by flagellate spines. The definition of the subgenus Olivemysis is modified in order to include H. -

<I>Calanus Pacificus,</I> a Planktonic
BULLETIN OF MARINE SCIENCE, 43(3): 675--{j94, ]988 VARIABILITY AND POSSIBLE ADAPTIVE SIGNIFICANCE OF DIEL VERTICAL MIGRATION IN CALANUS PACIFICUS, A PLANKTONIC MARINE COPEPOD Bruce W Frost ABSTRACT Adult females of a population of Calanus pacificus inhabiting a temperate fjord, Dabob Bay (Washington), exhibited seasonal and interannual variability in diel vertical migration. This variation was unrelated to food availability, in situ growth rate of females, and thermal stratification of the water column. A model of population growth of C pacificus, utilizing a life table approach founded on current knowledge of relevant physiological processes, was used to predict growth rates of migratory and nonmigratory populations and to test the implications of several alternative hypotheses for the adaptive significance of diel vertical migration. It was found that hypotheses invoking metabolic advantages for diel migrants (i.e., that dieI vertical migration is a foraging strategy optimizing individual growth rate) cannot, even when properly recast in terms of population growth, account for observed migration behavior. Rather, the model suggested a significant causal role for mortality op- erating differentially on migratory and nonmigratory individuals. The observations of mi- gration behavior in Dabob Bay are consistent with results of the model of population growth, which point unambiguously toward predator avoidance as the major selective advantage of dieI vertical migration in C pac!ficus. Mounting circumstantial evidence and theoretical arguments implicate pre- dation as a major driving force for diel vertical migrations of planktonic animals inhabiting lakes and areas of the ocean where advective losses are negligible (Zaret and Suffern, 1976; Wright et al., 1980; Stich and Lampert, 1981; Ohman et aI., 1983; Fancett and Kimmerer, 1985; Gliwicz, 1986a; 1986b; Lampert, 1987). -

Revising the Definition of the Crustacean Seta and Setal
Blackwell Science, LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2004? 2004 142? 233252 Original Article DEFINITION OF CRUSTACEAN SETAE AND SETAL CLASSIFICATION SYSTEMA. GARM Zoological Journal of the Linnean Society, 2004, 142, 233–252. With 11 figures Revising the definition of the crustacean seta and setal classification systems based on examinations of the mouthpart setae of seven species of decapods A. GARM* Biological Institute, University of Copenhagen, Denmark Received July 2003; accepted for publication June 2004 The crustacean cuticle has numerous projections and some of these projections, the setae, have important mechan- ical as well as sensory functions. The setae display a wide diversity in their external morphology, which has led to great problems separating setae from other projections in the cuticle and problems in making a consistent classifi- cation system. Here, the cuticular projections on the mouthparts of seven species of decapods are examined by scan- ning and transmission electron microscopy. A new definition is given: a seta is an elongate projection with a more or less circular base and a continuous lumen; the lumen has a semicircular arrangement of sheath cells basally. From the details of the external morphology the mouthpart setae are divided into seven types: pappose, plumose, serrulate, serrate, papposerrate, simple and cuspidate setae, which are suggested to reflect mechanical functions and not evolutionary history. This classification system is compared with earlier systems. © 2004 The Linnean Society of London, Zoological Journal of the Linnean Society, 2004, 142, 233–252. ADDITIONAL KEYWORDS: functional morphology – mechanical function – setal types. INTRODUCTION paper, the most often used term is setae and it will therefore be used here.