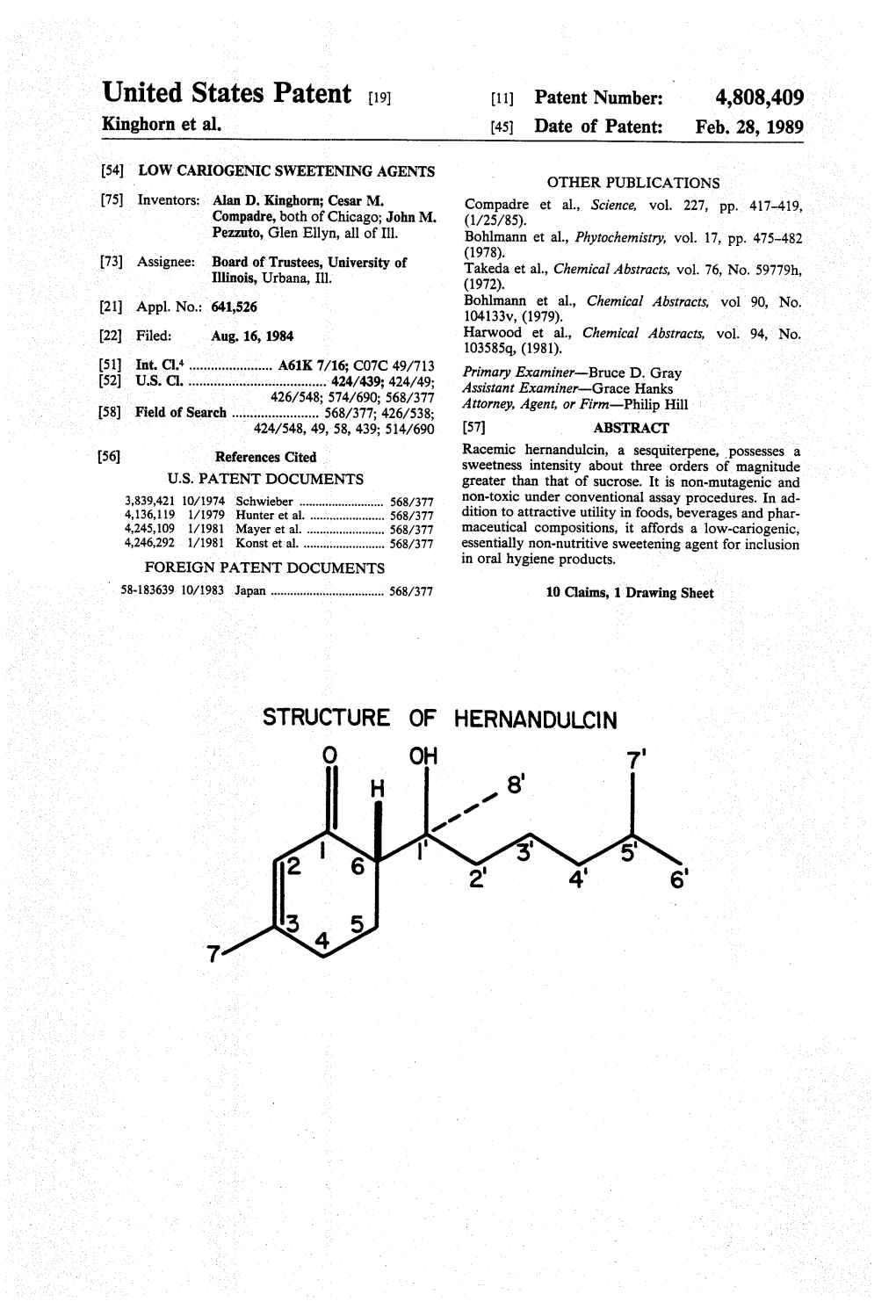
Hernandulcin, a Sesquiterpene, Possesses a [56] References Cited , Sweetness Intensity About Three Orders of Magnitude U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Popular Sweeteners and Their Health Effects Based Upon Valid Scientific Data
Popular Sweeteners and Their Health Effects Interactive Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science By __________________________________ Ivan Lebedev __________________________________ Jayyoung Park __________________________________ Ross Yaylaian Date: Approved: __________________________________ Professor Satya Shivkumar Abstract Perceived health risks of artificial sweeteners are a controversial topic often supported solely by anecdotal evidence and distorted media hype. The aim of this study was to examine popular sweeteners and their health effects based upon valid scientific data. Information was gathered through a sweetener taste panel, interviews with doctors, and an on-line survey. The survey revealed the public’s lack of appreciation for sweeteners. It was observed that artificial sweeteners can serve as a low-risk alternative to natural sweeteners. I Table of Contents Abstract .............................................................................................................................................. I Table of Contents ............................................................................................................................... II List of Figures ................................................................................................................................... IV List of Tables ................................................................................................................................... -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

JOURNAL of SCIENCE Published on Tho First Day of October, January, April and July
IOWA STATE COLLEGE JOURNAL OF SCIENCE Published on tho first day of October, January, April and July. EDITORIAL BOARD EDITOR-IN-CHIEF, R. E. Buchanan. ASSOCIATE EDITORS: P. E. Brown, Secretary; C. J. Drake, A H. Fuller, I. E. Melhus, E. A. Benbrook, P. Mabel Nelson, V. E. Nelson. ADVISORY EDITORS: R. E. Buchanan, Chairman; C. J. Drake, E. W. Lind strom, I. E. Melhus, P. Mabel Nelson, 0. R. Sweeney. BUSINESS CoMMITTEE: P. E. Brown, Chairman; A.H. Fuller, V: E. Nelson, Jay W. Woodrow. All manuscripts submitted for publication should be addressed to R. E. Buchanan, Room 101 Science Building, Iowa State College, Ames, Iowa_ All remittances should be addressed to P_ E. Brown, Bus. Mgr., Room 25 Hall of Agriculture, Iowa State College, Ames, Iowa. Single Copies: One Dollar; Annual Sub-scription Three Dollars; In Canada, Three Dollars and a Quarter; Foreign, Thre€ Dollars and a Half. ,..· ... J.. .:·::: . ... • . ... Entered as seco1.<u class matter.. at. ...... the . 0I'ost. 0 i!JtJ;;,. Ames, Iowa. .-·:.. ....·:::: :.·. .. ... : : :. ··: : . : ·... : .... ·.. ··: .··.··.: ~ ·. ·.. · ..... .·. ... : . .. .·. ...... .·.... THE QUANTITATIVE DETERMINATION OF FORMIC ACID IN THE PRE:SENCE OF ACETIC ACID BY A CONDUCTOMETRIC TITRATION• BY EDMOND E. MOORE WITH ELLIS I. FULMER. From the laboratory of biophysical chemistry, Iowa State College. Accepted for publication Juiie 3, 1929. OUTLINE I. Introduction. II. Review of quantitative methods for the determination of formic acid. III. Theoretical discussion of conductometric titration. IV. Equipment and procedure. V. Experimental results. VI. The use of the curves and diagram in analysis. VII. Summary. VIII. Literature cited. I. INTRODUCTION Quantitative studies of the chemical activities of microorganisms are at present handicapped by the lack of adequate analytical methods. -

Assessing and Evaluating Biomarkers and Chemical Markers by Targeted and Untargeted Mass Spectrometry-Based Metabolomics
MIAMI UNIVERSITY The Graduate School Certificate for Approving the Dissertation We hereby approve the Dissertation of Kundi Yang Candidate for the Degree Doctor of Philosophy ______________________________________ Dr. Michael Crowder, Director ______________________________________ Dr. Neil Danielson, Reader ______________________________________ Dr. Rick Page, Reader ______________________________________ Dr. Scott Hartley, Reader ______________________________________ Dr. Xin Wang, Graduate School Representative ABSTRACT ASSESSING AND EVALUATING BIOMARKERS AND CHEMICAL MARKERS BY TARGETED AND UNTARGETED MASS SPECTROMETRY-BASED METABOLOMICS by Kundi Yang The features of high sensitivity, selectivity, and reproducibility make liquid chromatography coupled mass spectrometry (LC-MS) the mainstream analytical platform in metabolomics studies. In this dissertation work, we developed two LC-MS platforms with electrospray ionization (ESI) sources, including a LC-triple quadrupole (LC-QQQ) MS platform for targeted metabolomics analysis and a LC-Orbitrap MS platform for untargeted metabolomics analysis, to assess and evaluate either biomarkers or chemical markers in various sample matrices. The main focus of targeted metabolomics platform was to study human gut microbiota and how the gut microbiome responds to therapies. We utilized a LC-QQQ targeted platform for this focus and conducted three projects: (1) to evaluate the effects of four nutrients, mucin, bile salts, inorganic salts, and short chain fatty acids, on the gut microbiome in terms -

Food Laboratory Services Guide
Food Laboratory Services Guide FDA Import Detention Services Food Adulteration, GMO & Quality Control Food Allergens Food Chemistry Food Microbiology Natural Toxins Nutritional Analysis Packaging Analysis Shelf Life Studies Specialty Chemistry Analysis Vitamins & Minerals The EMSL Diamond Standard EMSL Analytical, Inc., (EMSL) is a national network of laboratories located in key cities and regions nationwide and Canada. Established in 1981, the company has expanded its analytical services and ca- pabilities and now operates more than thirty-one locations all striving for excellence in providing quality laboratory services in a timely and cost competitive manner. Our diverse staff of over 585 employees includes a wide range of expertise, educational background and capabilities. These dedicated and capable employees follow the lead and standard of care demon- strated by the owner and founder of the company, Dr. Peter Frasca, who, as a hands on owner maintains daily involvement in our laboratory operations, and dictates that our work is consistent with his EMSL Diamond Standard. This “Diamond Standard” includes the following: u Quality Data - Strict Adherence to our Quality programs and regulatory requirements which comply with the ISO 17025 guidelines so that our data is tracked, managed, reported, and verified to be accurate and reliable. u Customer Dedication - We strive to create lasting, mutually beneficial relationships with all clients. We solicit feedback from our clients and we are committed to responding quickly to any questions or concerns that may arise before, during, or after an assignment. u Analytical Expertise - We employ highly qualified and experienced chemists, geologists, physicists, mycologists, microbiologists, biologists, materials scientists and industrial hygienists to enhance our analytical abilities and expertise. -

Recent Analytical Methods for the Analysis of Sweeteners in Food: a Regulatory Perspective Romina Shah U.S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Food and Drug Administration Papers U.S. Department of Health and Human Services 2017 Recent Analytical Methods for the Analysis of Sweeteners in Food: A Regulatory Perspective Romina Shah U.S. Food and Drug Administration, [email protected] Lowri S. de Jager U.S. Food and Drug Administration Follow this and additional works at: http://digitalcommons.unl.edu/usfda Part of the Dietetics and Clinical Nutrition Commons, Health and Medical Administration Commons, Health Services Administration Commons, Pharmaceutical Preparations Commons, and the Pharmacy Administration, Policy and Regulation Commons Shah, Romina and de Jager, Lowri S., "Recent Analytical Methods for the Analysis of Sweeteners in Food: A Regulatory Perspective" (2017). Food and Drug Administration Papers. 5. http://digitalcommons.unl.edu/usfda/5 This Article is brought to you for free and open access by the U.S. Department of Health and Human Services at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Food and Drug Administration Papers by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 2 Recent Analytical Methods for the Analysis of Sweeteners in Food: A Regulatory Perspective Romina Shah* and Lowri S. de Jager U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, College Park, Maryland, USA Abstract Non-nutritive or low calorie sweeteners are commonly used worldwide in the food industry, oftft en in combination in order to limit undesirable tastes. Th e list of allowable sweeteners varies among countries and it is important for regulatory agencies and food safety laboratories to monitor these highly consumed products to ensure compliance with worldwide regulations. -

17IFM07 Understanding Food Labels Final.Indd
Understanding Food Labels Understanding food labels makes it easier to stay on track with your health and nutrition goals, but the information found on food labels can be confusing. To add to the confusion, the U.S. Food and Drug Administration revised its rules for food labeling in 2016, giving manufacturers until July 2018 to comply with the rules. The current label is on the left, while the new label is on the right. As a consumer, you will likely see both of these food labels on packaged goods until July 2018. This handout walks you through the steps of reading both of these food labels. 1 1 2 2 3 3 4 4 1 This section of a food label contains information about the amount of food in the package. Labels indicate two things: the number of servings in the entire package, and the average serving size. Serving sizes are shown in two measurements: standard (cups) and metric (grams). In this example, the package contains 8 servings of food. One serving is equal to ⅔ cup, or 55 grams. 2 This section contains information about the number of calories in one serving of this food. Calories are units of energy generated by the food. Calories from fat are shown on old labels to indicate how much of the energy in each serving of food comes from fat. 3 Nutritional content is listed next. Figures are shown in grams for total fat, cholesterol, sodium, total carbohydrates, and protein. Some of these nutrient categories have subcategories. Under fat, two categories are listed: n Saturated fat: Saturated fat was once thought to be harmful, but current evidence suggests that it is not as bad as it once seemed. -

Downloaded Bulk Rnaseq Data [Four Samples for Each of Strain (129S1/Svimj and C57bl/6J)
bioRxiv preprint doi: https://doi.org/10.1101/379347; this version posted August 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 2 3 4 Genetic controls of mouse Tas1r3-independent sucrose 5 intake 6 7 8 Cailu Lin1, Michael G. Tordoff1, Xia Li1,2, Natalia P. Bosak1, Masashi Inoue1,3, Yutaka 9 Ishiwatari1,4, Gary K. Beauchamp1, Alexander A. Bachmanov1,5*, and Danielle R. Reed1* 10 1Monell Chemical Senses Center, Philadelphia, Pennsylvania, United States of America 11 2Current address: Sonora Quest Laboratories, Phoenix, Arizona, United States of America 12 3Current address: Tokyo University of Pharmacy and Life Science, Hachioji, Tokyo, Japan 13 4Current address: Ajinomoto Co., Inc., Tokyo, Japan 14 5Current address: GlaxoSmithKline, Collegeville, Pennsylvania, United States of America 15 *Corresponding author 16 E-mail: [email protected] (DRR) 17 18 19 20 bioRxiv preprint doi: https://doi.org/10.1101/379347; this version posted August 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 21 Acknowledgments 22 We gratefully acknowledge Maria L. Theodorides, Zakiyyah Smith, Mauricio Avigdor, and Amy 23 Colihan for assistance with animal breeding. We also acknowledge Richard Copeland and the 24 consistent high-quality assistance of the animal care staff at the Monell Chemical Senses 25 Center and thank them for their service. -
![Pdf [Accessed 05.06.13] [In Chinese]](https://docslib.b-cdn.net/cover/3435/pdf-accessed-05-06-13-in-chinese-2833435.webp)
Pdf [Accessed 05.06.13] [In Chinese]
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector journal of food and drug analysis 22 (2014) 318e328 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.jfda-online.com Original Article Detection of 10 sweeteners in various foods by liquid chromatography/tandem mass spectrometry Chui-Shiang Chang a, Tai Sheng Yeh b,* a Public Health Bureau, Pingtung County Government, Pingtung, Taiwan, ROC b Department of Food Science and Nutrition, Meiho University, Neipu Township, Pingtung, Taiwan, ROC article info abstract Article history: The analytical method for sweeteners in various food matrixes is very important for food Received 13 June 2013 quality control and regulation enforcement. A simple and rapid method for the simulta- Received in revised form neous determination of 10 sweeteners [acesulfame potassium (ACS-K), aspartame (ASP), 29 October 2013 cyclamate (CYC), dulcin (DUL), glycyrrhizic acid (GA), neotame (NEO), neohesperidin Accepted 27 December 2013 dihydrochalcone (NHDC), saccharin (SAC), sucralose (SCL), and stevioside (STV)] in various Available online 20 February 2014 foods by liquid chromatography/tandem mass chromatography (LCeMS/MS) was devel- oped. The chromatographic separation was performed on a Phenomenex Luna Phenyl- Keywords: Hexyl (5 mm, 4.6 mm  150 mm) column with gradient elution of 10 mM ammonium Beverages acetate in water and 10 mM ammonium acetate in methanol. The recoveries of the 10 Candied fruit sweeteners were between 75% and 120%, and the coefficients of variation were less than LCeMS/MS 20%. The limits of quantification were 0.5 mg/kg for NHDC and SCL. -

Emil Fischer Papers, 1876-1919
http://oac.cdlib.org/findaid/ark:/13030/tf6000053v No online items Finding Aid to the Emil Fischer Papers, 1876-1919 Processed by The Bancroft Library staff The Bancroft Library. University of California, Berkeley Berkeley, California, 94720-6000 Phone: (510) 642-6481 Fax: (510) 642-7589 Email: [email protected] URL: http://bancroft.berkeley.edu © 1996 The Regents of the University of California. All rights reserved. Note This finding aid has been fimed for the National Inventory of Documentary Sources in the United States (Chadwyck-Healey Inc.) Finding Aid to the Emil Fischer BANC MSS 71/95 z 1 Papers, 1876-1919 Finding Aid to the Emil Fischer Papers, 1876-1919 Collection number: BANC MSS 71/95 z The Bancroft Library University of California, Berkeley Berkeley, California Contact Information: The Bancroft Library. University of California, Berkeley Berkeley, California, 94720-6000 Phone: (510) 642-6481 Fax: (510) 642-7589 Email: [email protected] URL: http://bancroft.berkeley.edu Processed by: The Bancroft Library staff Date Completed: ca. 1971 Encoded by: Xiuzhi Zhou © 1996 The Regents of the University of California. All rights reserved. Collection Summary Collection Title: Emil Fischer Papers, Date (inclusive): 1876-1919 Collection Number: BANC MSS 71/95 z Creator: Fischer, Emil, 1852-1919 Extent: Number of containers: 39 boxes, 12 cartons, 7 oversize folders, 15 oversize volumes Repository: The Bancroft Library Berkeley, California 94720-6000 Physical Location: For current information on the location of these materials, please consult the Library's online catalog. Abstract: Correspondence; manuscripts, including drafts of his autobiography; reprints of his writings; subject files relating to his research and to work during World War I, and to professional activities; laboratory notebooks, his own and those of his students; clippings; photographs; and certificates of election or appointment to scientific societies. -

Positive Allosteric Modulators of the Human Sweet Taste Receptor Enhance Sweet Taste
Positive allosteric modulators of the human sweet taste receptor enhance sweet taste Guy Servanta,1,2, Catherine Tachdjiana,1, Xiao-Qing Tanga, Sara Wernera, Feng Zhanga, Xiaodong Lia, Poonit Kamdara, Goran Petrovica, Tanya Ditschuna, Antoniette Javaa, Paul Brusta, Nicole Brunea, Grant E. DuBoisb, Mark Zollera, and Donald S. Karanewskya aSenomyx, Inc., La Jolla, CA 92121; and bGlobal Research and Technology, The Coca-Cola Company, Atlanta, GA 30301 Edited* by Solomon Snyder, The Johns Hopkins University School of Medicine, Baltimore, MD, and approved January 25, 2010 (received for review October 12, 2009) To identify molecules that could enhance sweetness perception, concentrations (4, 5) and therefore potentially improving the we undertook the screening of a compound library using a cell- palatability and flavor profile of several consumer products. based assay for the human sweet taste receptor and a panel of Sweet taste is mediated by an obligate heterodimeric receptor selected sweeteners. In one of these screens we found a hit, SE-1, composed of two distinct subunits (G protein-coupled receptors, which significantly enhanced the activity of sucralose in the assay. GPCRs), T1R2 and T1R3, located at the surface of taste At 50 μM, SE-1 increased the sucralose potency by >20-fold. On receptor cells in the taste buds (6, 7). These subunits, members the other hand, SE-1 exhibited little or no agonist activity on its of family C GPCRs, possess a large extracellular N-terminal own. SE-1 effects were strikingly selective for sucralose. Other domain, the Venus flytrap domain (VFT), linked to the seven- popular sweeteners such as aspartame, cyclamate, and saccharin transmembrane C-terminal domain (TMD) by a shorter cys- were not enhanced by SE-1 whereas sucrose and neotame potency teine-rich domain (6–8). -

Analytical Methods for Determination of Non-Nutritive Sweeteners in Foodstuffs
molecules Review Analytical Methods for Determination of Non-Nutritive Sweeteners in Foodstuffs Viki Oktavirina 1, Nadhila B. Prabawati 1 , Rohmah Nur Fathimah 1, Miguel Palma 2 , Kiki Adi Kurnia 3, Noviyan Darmawan 4, Brian Yulianto 5,6 and Widiastuti Setyaningsih 1,* 1 Department of Food and Agricultural Product Technology, Faculty of Agricultural Technology, Gadjah Mada University, Jalan Flora No. 1, Bulaksumur, Sleman 55281, Indonesia; [email protected] (V.O.); [email protected] (N.B.P.); [email protected] (R.N.F.) 2 Department of Analytical Chemistry, Faculty of Sciences, IVAGRO, Campus de Excelencia Internacional Agroalimentario (CeiA3), Campus del Rio San Pedro, University of Cadiz, Puerto Real, 11510 Cadiz, Spain; [email protected] 3 Department of Marine, Faculty of Fisheries and Marine, Kampus C Jalan Mulyorejo, Universitas Airlangga, Surabaya 60115, Indonesia; [email protected] 4 Department of Chemistry, IPB University, IPB Dramaga, Bogor 16880, Indonesia; [email protected] 5 Department of Engineering Physics, Institut Teknologi Bandung, Jl. Ganesha 10, Bandung 40132, Indonesia; [email protected] 6 Research Center for Nanoscience and Nanotechnology (RCNN), Institut Teknologi Bandung, Jl. Ganesha 10, Bandung 40132, Indonesia * Correspondence: [email protected]; Tel.: +62-8211-319-0088 Abstract: Sweeteners have been used in food for centuries to increase both taste and appearance. However, the consumption of sweeteners, mainly sugars, has an adverse effect on human health Citation: Oktavirina, V.; Prabawati, when consumed in excessive doses for a certain period, including alteration in gut microbiota, N.B.; Fathimah, R.N.; Palma, M.; obesity, and diabetes.