Landscape Ecological Vegetation Map of St. Maarten (Lesser Antilles)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nature Policy Plan
Ministry of Public Housing, Spatial Planning, Environment And Infrastructure Ministerie van Volkshuisvesting, Ruimtelijke Ordening, Milieu en Infrastructuur Nature Policy Plan 2021 – 2025 Established/Approved: Date: By: i Nature Policy Plan Sint Maarten 2021 – 2025 “We the people of Sint Maarten: RESOLVED to provide for the continuing preservation of nature and the environment”. Constitution of Sint Maarten ii Nature Policy Plan Sint Maarten 2021 – 2025 Nature Policy Plan Sint Maarten 2021 – 2025 Ministry of Public Housing, Spatial Planning, Environment and Infrastructure (Ministry of VROMI) Address: Government of Sint Maarten Ministry of VROMI Soualiga Road #1 Pond Island, Great Bay Sint Maarten Contact: [email protected] [email protected] iii Nature Policy Plan Sint Maarten 2021 – 2025 Lignum Vitae (Guaiacum officinale) iv Photo by: Mark Yokoyama Nature Policy Plan Sint Maarten 2021 – 2025 Acknowledgments In writing the Nature Policy Plan Sint Maarten 2021 – 2025, the Ministry of VROMI consulted several government ministries, and external stakeholders including private sector entities and NGO’s. Some were engaged in the preparation of the policy from the onset; others were part of a review of the policy and stakeholder meetings. The Ministry of VROMI acknowledges and appreciates the time and effort of the stakeholders who contributed to the formulation of this Nature Policy Plan, which provides insights into the current state of affairs of nature on Sint Maarten and the proposed way forward on nature conservation -

Eye of the Wind Segeltoern
Fri 02 March 2018 - Tue 13 March 2018 Since the time of Christopher Columbus, the Bermuda Triangle – the mysterious region in the Atlantic Ocean between Miami, Puerto Rico and Bermuda – has been the subject of 'sailor's yarn', which sometimes tells stories of ships and planes that have disappeared without a trace, and of alleged hauntings or inexplicable natural phenomena. However, maritime superstition is not in our luggage on this crossing, as the Eye of the Wind will not be sailing into the legendary triangle, but instead only into the northern boundary point – the dreamlike Bermuda archipelago. This is where you will go on board: Marina Fort Louis, Marigot / Saint Martin The Princess Juliana international airport is located in the Dutch southern half of the twin island of Sint Maarten / Saint Martin and is easily reached from many major airports. A unique attraction worth seeing is immediately visible on anchoring at the Caribbean island paradise: on the nearby Maho Beach, the distance between landing airplanes and beach-goers' heads (or the masts of the boats anchored there) is often only a few metres. On a chalkboard attached to a surfboard, the arrival times of the largest planes are announced for hobby photographers every day. You can reach Marigot by taxi or minibus with a short drive. By the way, the EURO is the official currency in the French part of Saint Martin. Take advantage of your stay for a relaxing day at the breathtaking Dawn Beach. Our tip: Saint Martin is considered the 'culinary capital of the Caribbean' – do not miss the local specialties made from the guava fruit. -

St. Eustatius (Statia)
St. Eustatius (Statia) Overview: Sint Eustatius, also known as Statia, is one of the islands which make up the Netherlands Antilles. Over its long history, it changed hands more than 20 times. The island has remained in Dutch hands since 1816. Vocanic in origin, and quite rugged along the coastal areas, it was once one of the busiest and most powerful commercial ports in the Caribbean. As a result of the British blockade during America's Revolutionary War, most goods destined for the new colonies flowed into St. Eustatius. It was not uncommon to see hundreds of supply ships off Oranjestad. When that war ended and mail and supplies were sent directly to the new United States, the economy of St. Eustatius changed dramatically. According to tradition, it was the first foreign port to salute (1776) the American flag. Tourism is the major industry today, and the island is a favorite destination for scuba diving aficionados and nature lovers. Territory: Highest point = Mt. Mazinga, 1,968 ft. (602m) St. Eustatius is hilly with a central flat plain. Sandy beaches are rare. Geographically, the island is saddle- shaped, with the 602 meter-high Mount Mazinga, colloquially called the Quill (a dormant volcano) to the southeast and the smaller pair Signal Hill/Little Mountain and Boven Mountain to the northwest. The bulk of the island's population lives in the "dip" between the two areas, which crosses the center of the island. The island is just 5 miles long and 2 miles wide; area: 21 sq. km. Location: It is in the northern, Leeward Islands portion of the West Indies, some distance southeast of the Virgin Islands. -

'Good Governance' in the Dutch Caribbean
Obstacles to ‘Good Governance’ in the Dutch Caribbean Colonial- and Postcolonial Development in Aruba and Sint Maarten Arxen A. Alders Master Thesis 2015 [email protected] Politics and Society in Historical Perspective Department of History Utrecht University University Supervisor: Dr. Auke Rijpma Internship (BZK/KR) Supervisor: Nol Hendriks Introduction .............................................................................................................................. 2 1. Background ............................................................................................................................ 9 1.1 From Colony to Autonomy ......................................................................................................... 9 1.2 Status Quaestionis .................................................................................................................... 11 Colonial history .............................................................................................................................. 12 Smallness ....................................................................................................................................... 16 2. Adapting Concepts to Context ................................................................................................. 19 2.1 Good Governance ..................................................................................................................... 19 Development in a Small Island Context ........................................................................................ -

The Value of Nature in the Caribbean Netherlands
The Economics of Ecosystems The value of nature and Biodiversity in the Caribbean Netherlands in the Caribbean Netherlands 2 Total Economic Value in the Caribbean Netherlands The value of nature in the Caribbean Netherlands The Challenge Healthy ecosystems such as the forests on the hillsides of the Quill on St Eustatius and Saba’s Mt Scenery or the corals reefs of Bonaire are critical to the society of the Caribbean Netherlands. In the last decades, various local and global developments have resulted in serious threats to these fragile ecosystems, thereby jeopardizing the foundations of the islands’ economies. To make well-founded decisions that protect the natural environment on these beautiful tropical islands against the looming threats, it is crucial to understand how nature contributes to the economy and wellbeing in the Caribbean Netherlands. This study aims to determine the economic value and the societal importance of the main ecosystem services provided by the natural capital of Bonaire, St Eustatius and Saba. The challenge of this project is to deliver insights that support decision-makers in the long-term management of the islands’ economies and natural environment. Overview Caribbean Netherlands The Caribbean Netherlands consist of three islands, Bonaire, St Eustatius and Saba all located in the Caribbean Sea. Since 2010 each island is part of the Netherlands as a public entity. Bonaire is the largest island with 16,000 permanent residents, while only 4,000 people live in St Eustatius and approximately 2,000 in Saba. The total population of the Caribbean Netherlands is 22,000. All three islands are surrounded by living coral reefs and therefore attract many divers and snorkelers. -

Preliminary Checklist of Extant Endemic Species and Subspecies of the Windward Dutch Caribbean (St
Preliminary checklist of extant endemic species and subspecies of the windward Dutch Caribbean (St. Martin, St. Eustatius, Saba and the Saba Bank) Authors: O.G. Bos, P.A.J. Bakker, R.J.H.G. Henkens, J. A. de Freitas, A.O. Debrot Wageningen University & Research rapport C067/18 Preliminary checklist of extant endemic species and subspecies of the windward Dutch Caribbean (St. Martin, St. Eustatius, Saba and the Saba Bank) Authors: O.G. Bos1, P.A.J. Bakker2, R.J.H.G. Henkens3, J. A. de Freitas4, A.O. Debrot1 1. Wageningen Marine Research 2. Naturalis Biodiversity Center 3. Wageningen Environmental Research 4. Carmabi Publication date: 18 October 2018 This research project was carried out by Wageningen Marine Research at the request of and with funding from the Ministry of Agriculture, Nature and Food Quality for the purposes of Policy Support Research Theme ‘Caribbean Netherlands' (project no. BO-43-021.04-012). Wageningen Marine Research Den Helder, October 2018 CONFIDENTIAL no Wageningen Marine Research report C067/18 Bos OG, Bakker PAJ, Henkens RJHG, De Freitas JA, Debrot AO (2018). Preliminary checklist of extant endemic species of St. Martin, St. Eustatius, Saba and Saba Bank. Wageningen, Wageningen Marine Research (University & Research centre), Wageningen Marine Research report C067/18 Keywords: endemic species, Caribbean, Saba, Saint Eustatius, Saint Marten, Saba Bank Cover photo: endemic Anolis schwartzi in de Quill crater, St Eustatius (photo: A.O. Debrot) Date: 18 th of October 2018 Client: Ministry of LNV Attn.: H. Haanstra PO Box 20401 2500 EK The Hague The Netherlands BAS code BO-43-021.04-012 (KD-2018-055) This report can be downloaded for free from https://doi.org/10.18174/460388 Wageningen Marine Research provides no printed copies of reports Wageningen Marine Research is ISO 9001:2008 certified. -

St. Maarten – Netherlands Antilles)
The URBAN HERITAGE of PHILIPSBURG (St. Maarten – Netherlands Antilles) History of Foundation and Development & Report of Fieldwork by D. Lesterhuis & R. van Oers DELFT UNIVERSITY of TECHNOLOGY February 2001 Report in Commission of Dr. Shuji FUNO, Kyoto University - Japan O, sweet Saint Martin’s land, So bright by beach and strand, With sailors on the sea And harbours free. Where the chains of mountains green, Variously in sunlight sheen. O, I love thy paradise Nature-beauty fairily nice! O, I love thy paradise Nature-beauty fairily nice! Chorus of O Sweet Saint Martin’s Land, composed by G. Kemps in 1959. 2 Foreword Contents Within the Faculty of Architecture of Delft University of Technology the Department of Architectural Foreword Design/Restoration, chaired by Professor Dr. Frits van Voorden, has been conducting research into the characteristics, typologies and developments of Dutch overseas built heritage since the eighties Introduction of the last century. Traditional regions of study were the former colonies of the Netherlands. Because of close cultural-historic and political links and abundance in colonial architectural buildings and ensembles, an emphasis existed on the countries of Indonesia, Suriname, the Netherlands Chapter 1. General Overview and Short History Antilles and Sri Lanka. With the doctoral research of Van Oers, entitled Dutch Town Planning Overseas during VOC and • Dutch Presence in the West WIC Rule (1600-1800), the field of research of ‘mutual heritage’ was expanded to other regions • Principal Dutch Settlements in the West Indies: Willemstad & Philipsburg where the Dutch had been active in the planning and building of settlements. During that period new partnerships for co-operation in research were developed, of which the Graduate School of Engineering of Kyoto University in Japan is an important one. -

Hurricane & Tropical Storm
5.8 HURRICANE & TROPICAL STORM SECTION 5.8 HURRICANE AND TROPICAL STORM 5.8.1 HAZARD DESCRIPTION A tropical cyclone is a rotating, organized system of clouds and thunderstorms that originates over tropical or sub-tropical waters and has a closed low-level circulation. Tropical depressions, tropical storms, and hurricanes are all considered tropical cyclones. These storms rotate counterclockwise in the northern hemisphere around the center and are accompanied by heavy rain and strong winds (NOAA, 2013). Almost all tropical storms and hurricanes in the Atlantic basin (which includes the Gulf of Mexico and Caribbean Sea) form between June 1 and November 30 (hurricane season). August and September are peak months for hurricane development. The average wind speeds for tropical storms and hurricanes are listed below: . A tropical depression has a maximum sustained wind speeds of 38 miles per hour (mph) or less . A tropical storm has maximum sustained wind speeds of 39 to 73 mph . A hurricane has maximum sustained wind speeds of 74 mph or higher. In the western North Pacific, hurricanes are called typhoons; similar storms in the Indian Ocean and South Pacific Ocean are called cyclones. A major hurricane has maximum sustained wind speeds of 111 mph or higher (NOAA, 2013). Over a two-year period, the United States coastline is struck by an average of three hurricanes, one of which is classified as a major hurricane. Hurricanes, tropical storms, and tropical depressions may pose a threat to life and property. These storms bring heavy rain, storm surge and flooding (NOAA, 2013). The cooler waters off the coast of New Jersey can serve to diminish the energy of storms that have traveled up the eastern seaboard. -

East Coast of Mexico – 2018
East Coast of Mexico – 2018 Höegh Autoliners is one of the world’s leading Ro/Ro operators, carrying close to two million standard car units annually worldwide. Höegh Autoliners has transportation contracts with many of the world’s vehicle manufacturers and is in addition a leading carrier of second-hand vehicles as well as high and heavy construction equipment and other rolling stock. Our Pure Car/Truck Carrier (PCTC) service to and from East Coast of Mexico is operated by one of the most modern and flexible Ro/Ro fleets in the market. East Coast of Mexico Service Cargo Höegh Autoliners offers import and export possibilities via our regular ports Höegh Autoliners has for many years built a strong relationship with leading on the East Coast of Mexico: Veracruz and Altamira. With growth in the area car manufacturers and importers, for whom we ship new vehicles in different we have added connections to and from Latin American and Caribbean trade patterns worldwide. In addition to that, we focus strongly on the High ports. and Heavy and Breakbulk segments, where our professional staff and modern vessels are ready to cater for a variety of cargo. We offer a wide network of connections with around 100 ports linked with Veracruz and Altamira. We connect the East Coast of Mexico with USA, Latin Our sophisticated Ro/Ro vessels offer up to 6.5 meters of free deck height America and Caribbean, Europe, Middle East, Africa, India, East Asia, and can cater for cargo weighing up to 375 tonnes. The vessels are able to Oceania and South East Asia. -
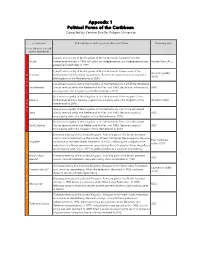
Appendix 1 Political Forms of the Caribbean Compiled by Yarimar Bonilla, Rutgers University
Appendix 1 Political Forms of the Caribbean Compiled by Yarimar Bonilla, Rutgers University Jurisdiction Political Status and Important Historical Dates Monetary Unit * = on UN list of non-self- governing territories Constituent country of the Kingdom of the Netherlands. Seceded from the Aruba Netherlands Antilles in 1986 with plans for independence, but independence was Aruban florin (AFL) postponed indefinitely in 1994. Constituent country of the Kingdom of the Netherlands. Former seat of the Antillean guilder N Curacao Netherlands Antilles central government. Became an autonomous country within (ANG) E the kingdom of the Netherlands in 2010. T Constituent Country within the Kingdom of the Netherlands. Part of the Windward H Sint Maarten Islands territory within the Netherland Antilles until 1983. Became an autonomous ANG E country within the Kingdom of the Netherlands in 2010. R L Special municipality of the Kingdom of the Netherlands. Formerly part of the A Bonaire Netherlands Antilles. Became a special municipality within the Kingdom of the US dollar (USD) N Netherlands in 2010. D Special municipality of the Kingdom of the Netherlands. Part of the Windward S Saba Islands territory within the Netherland Antilles until 1983. Became a special USD municipality within the Kingdom of the Netherlands in 2010. Special municipality of the Kingdom of the Netherlands. Part of the Windward Sint Eustatius Islands territory within the Netherland Antilles until 1983. Became a special USD municipality within the Kingdom of the Netherlands in 2010 Overseas territory of the United Kingdom. Formerly part of the British Leeward Island colonial federation as the colony of Saint Cristopher-Nevis-Anguilla. -

Molecular and Morphological Support for a Florida Origin of the Cuban
Journal of Biogeography (J. Biogeogr.) (2011) SPECIAL Molecular and morphological support ISSUE for a Florida origin of the Cuban oak Paul F. Gugger* and Jeannine Cavender-Bares Department of Ecology, Evolution and ABSTRACT Behavior, University of Minnesota, 100 Ecology Aim The origins of the Cuban biota are of long-standing interest in Building, 1987 Upper Buford Circle, Saint Paul, MN, USA biogeography, and the source of a small live oak (Quercus series Virentes) population on Cuba remains unresolved. Based on morphological evidence, previous authors have hypothesized a Florida origin from either Q. geminata or Q. virginiana or both; a Mexican origin from Q. oleoides; or a hybrid origin from both sources. We use molecular data and taxonomically informative leaf morphology to identify the source species and timing of colonization. Location Cuba, Central America, Mexico and the south-eastern United States. Methods We collected representative samples of Cuban oaks and each putative source species and genotyped each sample at 12 nuclear microsatellites and two chloroplast DNA sequences. We estimated population structure using a Bayesian clustering analysis and F-statistics, pairwise migration rates among taxa, and divergence time using an isolation-with-migration model. We measured seven leaf traits and conducted an analysis of similarity (ANOSIM) to determine which putative source species was most similar to Cuban oaks. Results Cuban oak contains one chloroplast DNA haplotype, which is common in southern Florida. Bayesian clustering analysis of microsatellites revealed that the Cuban oak forms a distinct and pure population cluster, and F-statistics showed that Cuban oaks are differentiated least from Q. virginiana and most from Q. -

Listado De Todas Las Plantas Que Tengo Fotografiadas Ordenado Por Familias Según El Sistema APG III (Última Actualización: 2 De Septiembre De 2021)
Listado de todas las plantas que tengo fotografiadas ordenado por familias según el sistema APG III (última actualización: 2 de Septiembre de 2021) GÉNERO Y ESPECIE FAMILIA SUBFAMILIA GÉNERO Y ESPECIE FAMILIA SUBFAMILIA Acanthus hungaricus Acanthaceae Acanthoideae Metarungia longistrobus Acanthaceae Acanthoideae Acanthus mollis Acanthaceae Acanthoideae Odontonema callistachyum Acanthaceae Acanthoideae Acanthus spinosus Acanthaceae Acanthoideae Odontonema cuspidatum Acanthaceae Acanthoideae Aphelandra flava Acanthaceae Acanthoideae Odontonema tubaeforme Acanthaceae Acanthoideae Aphelandra sinclairiana Acanthaceae Acanthoideae Pachystachys lutea Acanthaceae Acanthoideae Aphelandra squarrosa Acanthaceae Acanthoideae Pachystachys spicata Acanthaceae Acanthoideae Asystasia gangetica Acanthaceae Acanthoideae Peristrophe speciosa Acanthaceae Acanthoideae Barleria cristata Acanthaceae Acanthoideae Phaulopsis pulchella Acanthaceae Acanthoideae Barleria obtusa Acanthaceae Acanthoideae Pseuderanthemum carruthersii ‘Rubrum’ Acanthaceae Acanthoideae Barleria repens Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. atropurpureum Acanthaceae Acanthoideae Brillantaisia lamium Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. reticulatum Acanthaceae Acanthoideae Brillantaisia owariensis Acanthaceae Acanthoideae Pseuderanthemum laxiflorum Acanthaceae Acanthoideae Brillantaisia ulugurica Acanthaceae Acanthoideae Pseuderanthemum laxiflorum ‘Purple Dazzler’ Acanthaceae Acanthoideae Crossandra infundibuliformis Acanthaceae Acanthoideae Ruellia