Hemolytic Anemias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemolysis and Venous Thrombosis: Which Link? A
ISSN: 2378-3656 Rkiouak et al. Clin Med Rev Case Rep 2020, 7:329 DOI: 10.23937/2378-3656/1410329 Volume 7 | Issue 11 Clinical Medical Reviews Open Access and Case Reports ORIGINAL RESEARCH Hemolysis and Venous Thrombosis: Which Link? A. Rkiouak, PH.D1, I El Kassimi, MD2, N Sahel, MD2, M Zaizaa, MD2 and Y Sekkach, PhD1 Check for updates Internal Medicine A Department, Mohammed V Military Hospital Medical School of Rabat, Morocco *Corresponding author: Adil Rkiouak, PH.D., Department of Internal Medicine A, Mohammed V Military Hospital, Medical School of Rabat, Morocco, Tel: +212-66-179-44-04 The mechanism of antibody-mediated hemolysis is Abstract via phagocytosis or complement-mediated destruction The association hemolysis and venous thrombosis remains and can occur intravascular or extravascular. The intra- unknown to clinicians, despite our advances in comrehen- sion of pathophysiological bases. vascular mechanisms include direct cellular destruction via lysis, toxins, or trauma; fragmentation and oxida- Haemolysis, which is observed in multiple diseases, can affect all three components of Virchow’s triad. It is not sur- tion. prising that there is a link between haemolytic disorders and Multiple haemolytic disorders produce substantial thrombosis. intravascular haemolysis. Examples, the corpuscular We will try to clarify the main pro-thrombotic mechanisms hemolysis include PNH, extra-corpuscular haemolysis, during hemolysis through 3 clinical observations of deep ve- acquired (autoimmune haemolytic anaemia (AIHA , nous thrombosis in 3 main types of hemolytic pathologies, ) namely a case of paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura (PTT)), as well thrombotic thrombocytopenic purpura and autoimmune as other diseases. These disorders are also associated anemia hemolytic. -

Clostridial Septicemia with Intravascular Hemolysis: a Case Report G
Henry Ford Hospital Medical Journal Volume 13 | Number 4 Article 4 12-1965 Clostridial Septicemia With Intravascular Hemolysis: A Case Report G. M. Mastio E. Morfin Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Mastio, G. M. and Morfin, E. (1965) "Clostridial Septicemia With Intravascular Hemolysis: A Case Report," Henry Ford Hospital Medical Bulletin : Vol. 13 : No. 4 , 421-425. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol13/iss4/4 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Bull. Vol. 13, December 1965 CLOSTRIDIAL SEPTICEMIA WITH INTRAVASCULAR HEMOLYSIS A CASE REPORT G. M. MASTIC, M.D. AND E. MORFIN, M.D. In 1871 Bottini' demonstrated the bacterial nature of gas gangrene, but failed to isolate a causal organism. Clostridium perfringens, sometimes known as Clostridium welchii, was discovered independently during 1892 and 1893 by Welch, Frankel, "Veillon and Zuber.^ This organism is a saprophytic inhabitant of the intestinal tract, and may be a harmless saprophyte of the female genital tract occurring in the vagina in 4-6 per cent of pregnant women. Clostridial organisms occur in great numbers and distribution throughout the world. Because of this, they are very common in traumatic wounds. Very few species of Clostridia, however, are pathogenic, and still fewer are capable of producing gas gangrene in man. -

Viral Hepatitis with Acute Hemoglobinuria
22 Case Report Viral Hepatitis with Acute Hemoglobinuria Jagabandhu Ghosh1 Joydeep Ghosh2 1 Department of Paediatrics, I.P.G.M.E.R and S.S.K.M Hospital, Kolkata, Address for correspondence Jagabandhu Ghosh, MD (PAED), Ushashi West Bengal, India Housing Society, 245 Vivekananda Road, Kolkata 700006, India 2 Department of Biotechnology, Heritage Institute of Technology, (e-mail: [email protected]). Kolkata, West Bengal, India J Pediatr Infect Dis 2015;10:22–24. Abstract A 7-year-old male child presented with moderate degree fever, yellowish discoloration of eyes and urine. Examination on admission revealed severe anemia, jaundice, hepato- Keywords megaly, and splenomegaly. On the day following admission, the child showed evidence ► hepatitis of blackish discoloration of urine. The diagnosis was established as viral A hepatitis with ► G6PD glucose-6-phosphate dehydrogenase (G6PD) deficiency. The child recovered with ► viral supportive therapy. We suggest that either universal immunization against hepatitis ► hemolysis A, or routine newborn screening for G6PD deficiency, could prevent the serious ► intravascular morbidity or mortality that can occur when these two conditions coexist. Introduction of eyes, and urine for the past 5 days before admission. There was no history of hematemesis, melena, or any other bleed- Acute viral hepatitis A is widely prevalent in India.1 Viral ing, swelling of body, convulsion, and drug ingestion just hepatitis is the leading of acute hepatitis in children in our before present illness or any previous blood transfusion. His country, and hepatitis A is the most common type of viral urine volume was satisfactory. On examination, the child was hepatitis. Hepatitis A does not commonly present with severe deeply icteric, severely anemic, and drowsy. -
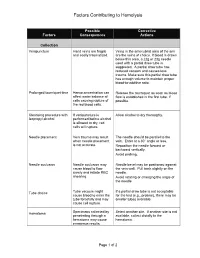
Factors Contributing to Hemolysis
Factors Contributing to Hemolysis Possible Corrective Factors Consequences Actions Collection Venipuncture Hand veins are fragile Veins in the antecubital area of the arm and easily traumatized. are the veins of choice. If blood is drawn below this area, a 22g or 23g needle used with a partial draw tube is suggested. A partial draw tube has reduced vacuum and causes less trauma. Make sure this partial draw tube has enough volume to maintain proper blood-to-additive ratio. Prolonged tourniquet time Hemoconcentration can Release the tourniquet as soon as blood affect water balance of flow is established in the first tube, if cells causing rupture of possible. the red blood cells. Cleansing procedure with If venipuncture is Allow alcohol to dry thoroughly. isopropyl alcohol performed before alcohol is allowed to dry, red cells will rupture. Needle placement Vein trauma may result The needle should be parallel to the when needle placement vein. Enter at a 30° angle or less. is not accurate. Reposition the needle forward or backward vertically. Avoid probing. Needle occlusion Needle occlusion may Needle bevel may be positioned against cause blood to flow the vein wall. Pull back slightly on the slowly and initiate RBC needle. shearing. Avoid rotating or changing the angle of the needle. Tube choice Tube vacuum might If a partial draw tube is not acceptable cause blood to enter the for the test (e.g., protime), there may be tube forcefully and may smaller tubes available. cause cell rupture. Hematoma Specimens collected by Select another site. If another site is not penetrating through a available, collect distally to the hematoma may cause hematoma. -

The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: a Review
Journal of Clinical Medicine Review The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: A Review Felix Scholkmann 1,2,*, Tanja Restin 2, Marco Ferrari 3 and Valentina Quaresima 3 1 Biomedical Optics Research Laboratory, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland 2 Newborn Research Zurich, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland; [email protected] 3 Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; [email protected] (M.F.); [email protected] (V.Q.) * Correspondence: [email protected]; Tel.: +41-4-4255-9326 Abstract: Following the outbreak of a novel coronavirus (SARS-CoV-2) associated with pneumonia in China (Corona Virus Disease 2019, COVID-19) at the end of 2019, the world is currently facing a global pandemic of infections with SARS-CoV-2 and cases of COVID-19. Since severely ill patients often show elevated methemoglobin (MetHb) and carboxyhemoglobin (COHb) concentrations in their blood as a marker of disease severity, we aimed to summarize the currently available published study results (case reports and cross-sectional studies) on MetHb and COHb concentrations in the blood of COVID-19 patients. To this end, a systematic literature research was performed. For the case of MetHb, seven publications were identified (five case reports and two cross-sectional studies), and for the case of COHb, three studies were found (two cross-sectional studies and one case report). The findings reported in the publications show that an increase in MetHb and COHb can happen in COVID-19 patients, especially in critically ill ones, and that MetHb and COHb can increase to dangerously high levels during the course of the disease in some patients. -

A Suspected Case of Clostridium Perfringens Sepsis With
Uojima et al. Journal of Medical Case Reports (2019) 13:125 https://doi.org/10.1186/s13256-019-2023-x CASE REPORT Open Access A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report Haruki Uojima1,2*, Mie Onoue2, Hisashi Hidaka2, Naohisa Wada2, Yoshiaki Tanaka2, Tomoyoshi Inoue2, Kousuke Kubota2, Takahide Nakazawa2, Akitaka Shibuya2 and Wasaburo Koizumi2 Abstract Introduction: Sepsis due to Clostridium perfringens, one of several clostridial species, is an important cause of massive intravascular hemolysis in patients with underlying malignancies. Chronic liver diseases, immunosuppression, and presence of malignancies were risk factors for Clostridium perfringens sepsis. Therefore, Clostridium perfringens sepsis should always be considered in patients presenting with liver damage after chemo- embolic therapy for hepatocellular carcinoma. This case report focuses on findings characteristic of an intravascular hemolysis due to Clostridium perfringens after transhepatic arterial chemoembolization. Case presentation: An 83-year-old Japanese man presented to our hospital because of a third recurrence of hepatocellular carcinoma. He had nonalcoholic steatohepatitis-related cirrhosis, and underwent radiofrequency ablation and transhepatic arterial chemoembolization therapy for hepatocellular carcinoma of S4/S8 and S2. He had a medical history of pancreatic carcinoma and underwent pylorus-preserving pancreaticoduodenectomy approximately 5 years ago. Because follow-up -

The Lower Urinary Tract & Male Reproductive System
Chapter 21 Hematopathology Nam Deuk Kim, Ph.D. Pusan National University 1 Contents I. Red blood cells II. Hemostasis III. White blood cells IV. Disorders of the lymphopoietic system V. Spleen VI. Thymus 2 THE HEMATOPOIETIC SYSTEM Composition of Human Blood Blood • Transports oxygen, nutrients, hormones, leukocytes (white cells), red cells, platelets and antibodies to tissues in the body and carbon dioxide and other waste products of cell metabolism to the excretory organs of the body • Volume of blood: Represents about 8% of total body weight • approximately 5 quarts, but it varies according to size of individual (5 liters in women; 5.5 liters in men) • Almost half of the blood consists of cellular elements suspended in plasma (viscous fluid) 3 4 Hemopoiesis Cellular differentiation and maturation of the lymphoid and myeloid components of the hematopoietic system. Only the precursor cells (blasts and maturing cells) are identifiable by light microscopic evaluation of the bone marrow. BFU = burst-forming unit; CFU = colony-forming unit (Ba = basophils; E = erythroid; Eo = eosinophils; G = polymorphonuclear leukocytes; GM = granulocyte-monocyte; M = monocyte/macrophages; Meg = megakaryocytic); EPO = erythropoietin; Gm-CSF = granulocyte-macrophage colony-stimulating factor; IL = interleukin; NK = natural killer; SCF = stem cell factor; TPO = thrombopoietin. 5 Composition of Human Blood • All blood cells arise from precursor cells within the bone marrow, called stem cells • These undergo further differentiation to form red cells, white cells, -

Hematology Unit Lab 1 Review Material
Hematology Unit Lab 1 Review Material Objectives Laboratory instructors: 1. Facilitate lab discussion and answer questions Students: 1. Review the introductory material below 2. Study and review the assigned cases and questions in small groups before the Lab. This includes the pathological material using Virtual Microscopy 3. Be prepared to present your cases, questions and answers to the rest of your Lab class during the Lab Erythropoiesis: The process of red blood cell (RBC) production • Characterized by: − Increasing hemoglobin synthesis Erythroid maturation stages (Below): − Decreasing cell size - Average of 4 cell divisions during maturation − Decreasing cytoplasmic basophilia [One pronormoblast gives rise to 16 red cells] (increasing pink color) - pronormoblast → reticulocyte = 7 days − Progressive chromatin condensation of the - reticulocytes → mature RBC =1-2 days nuclei − Extrusion of nucleus (orthochromatic stage) − Extruded nuclei are subsequently phagocytized − Loss of mitotic capability after the early stage of polychromatophilic normoblast • Picture below: Erythroid progenitors (normoblasts) cluster around macrophages (arrows) in the bone marrow and spleen • Macrophages store iron • Iron is transferred from macrophages to erythroid precursor cells • Iron is used by normoblasts for hemoglobin synthesis aka nucleated rbc aka reticulocyte 1 Mature Red Blood Cell 7-8 microns; round / ovoid biconcave disc with orange-red cytoplasm, no RNA, no nucleus; survives ~120 days in circulation Classification of Anemia by Morphology 1. -

Hemophagocytic Lymphohistiocytosis in a Child with Sickle Cell Disease
Hematology & Transfusion International Journal Case Report Open Access Hemophagocytic lymphohistiocytosis in a child with sickle cell disease Keywords: hemophagocytic lymphohistiocytosis, FHL, EBV, hemoglobin S, sickle cell disease, X-ray Volume 6 Issue 5 - 2018 Introduction Walaa Shoman,1 Yasmine El Chazli,2 Asmaa Elsharkawy,2 Neveen Mikhael,3 Akram Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening deghaidy,3 Abeer Al-Battashi,4 Yasser Wali2 hyper-inflammatory syndrome which represents the extreme end of 1Department of Pediatrics, Immunology/Rheumatology unit, a severe uncontrolled hyperinflammatory reaction that can occur in Alexandria University, Egypt many underlying conditions.1 HLH can be divided into primary and 2Department of Pediatrics, Hematology/Oncology unit, secondary HLH. Primary HLH is caused by gene mutation, either at Alexandria University, Egypt 3 one of the Familial HLH (FHL) loci or in a gene responsible for one Department of Clinical pathology, Alexandria University, Egypt 4Department of Child Health, Royal Hospital, Oman of several immunodeficiency syndromes.2 The most common form of secondary HLH is infection associated HLH. Infectious triggers Correspondence: Yasser Wali, Department of Pediatrics, include viruses (as EBV, cytomegalovirus, HHV8, HIV), bacteria (as Hematology/Oncology unit, Alexandria University, Egypt, mycobacteria, mycoplasma), parasites (as leishmania, plasmodium), Email [email protected] 3,4 and fungi (as candida, cryptococcus). Sickle cell disease (SCD) Received: September 02, 2018 | Published: September 27, and its variants are genetic disorders resulting from the presence 2018 of a mutated form of hemoglobin, hemoglobin S (HbS), which can be detected by hemoglobin electrophoresis. SCD is suggested by a high-grade unremitting fever, grunting, marked pallor, jaundice, the typical clinical picture of chronic hemolytic anemia and vaso- and marked re-enlargement of both liver and spleen despite improved occlusive crisis.5,6 We present a case of HLH in an Egyptian boy who chest condition and radiogram. -

Hereditary Spherocytosis with Gilbert's Syndrome
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 20, Issue 4 Ser.6 (April. 2021), PP 25-28 www.iosrjournals.org Hereditary Spherocytosis with Gilbert’s Syndrome – a case report. Dr Deepan Panneerselvam Intern, Department of Internal Medicine. Dr Thabuna Sivaprakasam Intern, Department of Internal Medicine. Dr Raja sekar PGY2 Internal Medicine Resident. Dr Govindarajulu Ethirajulu Chief, Department of Internal Medicine. Government Kilpauk Medical College and Hospital. Abstract: A 23 year old previously diagnosed female with Gilbert’s syndrome on treatment with Prednisone, presented with a lower respiratory tract infection for the past 4 days with additional complaints of significant lethargy and fatigue, palpitation and intermittent yellowish discolouration of sclera since 9 years old. The patient’s mother and elder sister also had similar history of intermittent jaundice. Routine investigations of the patient revealed normal values except a hemoglobin level of 6.0 g/dl, HCT of 19.5%, MCV of 75.6 fL, serum total bilirubin of 6.3 mg/dl ( Direct: 1.3mgdl, Indirect: 5.0 mg/dl ). This made us suspect another basic hematologic abnormality that contributed to such elevated indirect bilirubin levels. Further investigations revealed a peripheral smear with moderate microcytic hypochromic anemia, anisopoikilocytosis, leucopenia and a reticulocyte count of 2.3%, negative Direct coombs test and a serum LDH of 345U/L. Ultrasound sound abdomen revealed multiple calculi in gall bladder largest measuring 3mm and splenomegaly. Peripheral smear was repeated only to reveal a dimorphic blood picture with eosinophilia, normocytic normochromic anemia with a few fragmented RBCs and spherocytes. -

Approach to Anemia
APPROACH TO ANEMIA Mahsa Mohebtash, MD Medstar Union Memorial Hospital Definition of Anemia • Reduced red blood mass • RBC measurements: RBC mass, Hgb, Hct or RBC count • Hgb, Hct and RBC count typically decrease in parallel except in severe microcytosis (like thalassemia) Normal Range of Hgb/Hct • NL range: many different values: • 2 SD below mean: < Hgb13.5 or Hct 41 in men and Hgb 12 or Hct of 36 in women • WHO: Hgb: <13 in men, <12 in women • Revised WHO/NCI: Hgb <14 in men, <12 in women • Scrpps-Kaiser based on race and age: based on 5th percentiles of the population in question • African-Americans: Hgb 0.5-1 lower than Caucasians Approach to Anemia • Setting: • Acute vs chronic • Isolated vs combined with leukopenia/thrombocytopenia • Pathophysiologic approach • Morphologic approach Reticulocytes • Reticulocytes life span: 3 days in bone marrow and 1 day in peripheral blood • Mature RBC life span: 110-120 days • 1% of RBCs are removed from circulation each day • Reticulocyte production index (RPI): Reticulocytes (percent) x (HCT ÷ 45) x (1 ÷ RMT): • <2 low Pathophysiologic approach • Decreased RBC production • Reduced effective production of red cells: low retic production index • Destruction of red cell precursors in marrow (ineffective erythropoiesis) • Increased RBC destruction • Blood loss Reduced RBC precursors • Low retic production index • Lack of nutrients (B12, Fe) • Bone marrow disorder => reduced RBC precursors (aplastic anemia, pure RBC aplasia, marrow infiltration) • Bone marrow suppression (drugs, chemotherapy, radiation) -

Laboratory Approach to Anemia Laboratory Approach to Anemia
DOI: 10.5772/intechopen.70359 Provisional chapter Chapter 12 Laboratory Approach to Anemia Laboratory Approach to Anemia Ebru Dündar Yenilmez and Abdullah Tuli Ebru Dündar Yenilmez and Abdullah Tuli Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.70359 Abstract Anemia is a major cause of morbidity and mortality worldwide and can be defined as a decreased quantity of circulating red blood cells (RBCs). The epidemiological studies suggested that one-third of the world’s population is affected with anemia. Anemia is not a disease, but it is instead the sign of an underlying basic pathological process. However, the sign may function as a compass in the search for the cause. Therefore, the prediag- nosis revealed by thorough investigation of this sign should be supported by laboratory parameters according to the underlying pathological process. We expect that this review will provide guidance to clinicians with findings and laboratory tests that can be followed from the initial stage in the anemia search. Keywords: anemia, complete blood count, red blood cell indices, reticulocyte 1. Introduction Anemia, the meaning of which in Greek is “without blood,” is a relatively common sign and symptom of various medical conditions. Anemia is defined as a significant decrease in the count of total erythrocyte [red blood cell (RBC)] mass, although this definition is rarely used in clinical settings. According to the World Health Organization, anemia is a condition in which the number of red blood cells (RBCs, and consequently their oxygen-carrying capacity) is insufficient to meet the body’s physiologic needs [1, 2].